Introduction
India fourth in the world in incidence of oral cancer with 9.1 per 100,000 people, after Papua New Guinea (20.4), Pakistan (12.2), and Bangladesh (9.5) (1). According to the report of the National Institute of Health and Family Welfare (NIHFW), the country had 86% of the world’s oral cancer cases. The rising curve of oral cancer is a major health hazard in India (2). One-fourth of Indian adults (23.9%) consume areca and among that 10% of the users consume it along with tobacco. With 223.79 million users, India tops globally both in the production and consumption of areca nuts (3). India protects the cultivation, and the areca nut production saw a rise from 2.5 lakh metric tons, of annual production in the 1990s, to 9 lakh metric tons in 2020. These earnings from areca nuts are much order than other cash crops (4). Areca nuts are cultivated in almost every individual house along the boundary walls (Figure 1) and areca is considered sacred according to cultural and religious beliefs (5). In states like Assam, Manipur, Meghalaya, Mizoram, and Uttar Pradesh, 40% of men consume areca nut in different forms. Northeastern states have a relatively higher prevalence of tobacco use along with areca nuts, whereas southern states consume betel quid (BQ) without tobacco (6). Uttar Pradesh reported 22% of all areca consumers followed by Maharashtra (12%), Karnataka (9%), and Tamil Nadu (8%). A demographic divide exists in the uses; education levels affected the usage and statistic shows that daily wage earners have the highest (30%) level of consumption; usage in the population belonging to the scheduled tribe was 25.8%, and in Muslims, 30.8% usage was noted (3). Areca nut is seldom used in pure form. Various combinations, additions, preparations, and processing are made (e.g., in gutka and paan masalas) and consumed alone or along with a BQ.

Figure 1. (A) Arrow points at the Areca fruits have the kernel inside which is consumed. (B) Both ripe (orange color) and unripe (green colour) varieties are seen on the same tree.
Despite its rampant use as a psychoactive agent, the areca nut and gutka chewing habit and addiction remain a gross under-researched area compared to the endemicity of its use. The western world cannot recognize the complexity and use of numerous components in these oral habits (7). This may be attributed to underreporting, linguistics, and social and cultural gaps (8, 9). This necessitates compiling the usage and forms in distinguishable groups while reporting or reviewing. Recent global reviews call for more research on areca consumption, which is the world’s fourth largest addicting agent (10). In this review, the misconception which assumes that areca nut/BQ are not carcinogenic without tobacco addition is dealt with.
Areca nut consumption: The variability quotients
Raw nuts may be consumed dried (sun-dried or freeze-dried) or fresh, mature or immature, or processed (soaked, boiled, or prepared with numerous additives) (11). Similarly, there are numerous variations in tobacco that are used in conjunction with areca nuts. It may be either dried mature leaves or boiled fermented leaves, or differently flavored processed leaves (e.g., in zarda); variations exist in the relative number and contents of different components, while they are prepared in paan shops (11, 12). Furthermore, the amount of chewing/grinding (time of oral exposure), consumption versus rejection (through spitting the oral contents), and extractions vary and so do the methods of consumption that affect the severity of afflictions (13).
Cocktail of a variety of mixtures in BQ and Paan Masala deterrent for definitive study
In low- and middle-income countries and in a heterogeneous population like ours, the multiplexity and variety of use of the chemicals and components (lacking labels), unregulated local manufacturing, and consumption make the whole thing virtually indistinguishable and confusing (Figures 2–4) (12). There is specificity in uses and damage. Damages created by the variety of individual components with numerous combinations make it more complex to report specifically (9). Here, it is worth mentioning that the addiction item preferences and habits are specific for an individual and are relatively constant and an attempt at generalization is a gross mistake. For reviewers, the problem for reporting and relating becomes manifold (14), with alterations of the pharmacology, variety of bio-availability of extracted phytochemicals, variations in mutual interactions of the chemical cocktails, and the extent of phytochemical extraction all being a deterrent in definitive reporting of damages created (15–18).

Figure 2. Different individual components are displayed in the paan shop. Metal-coated cardamom seeds (1), colored fennels (2) with original spices of cardamom (3A), cloves (3B), and fennel seeds (3C) are seen. Various colored vegetables (4) and locally made components are added.

Figure 3. The making of betel quid (BQ1, BQ2, and BQ3) are shown here. The components are labeled in the figures. The names of individual components of BQ are as follows:A – Betel leaf, B – Areca nuts, C – Slaked lime, D – Catechu, E – Zarda, F – Chaman Bahar, G – Colored vegetables, H – Gulkand, and I – Coated condiments of paan masala. Various zarda (E1-E4) are added as per preferences (anyone, E1-E4) and quantity is shown.

Figure 4. (A) Paan masala sachets are sold together (8 larger packs) containing 3–4 g of mixture with zarda. (B) (similar 8 smaller packs) of the same companies to make a combinatorial gutka mixture (zarda sachets are to the right of the paan masala packs). Labels are obfuscated. (C) Only 3 of them listed their ingredients where synthetic flavors are used as opposed to FSSAI guidelines. (D) All the paan masala packs and corresponding zarda packs are evacuated and the contents were displayed (inner ring is of zarda). In the center is shown dried areca nut (in raw pure form) of a particular cut as consumed or used to make BQ.
This article will be accounting for the various forms of areca consumption in paan masala, BQ (variations in preparations), and gutka, all of which contain areca nuts as the principal ingredient. The different uses of oral tobacco/smokeless tobacco forms such as in zarda and khaini which are used along with areca nuts or separately consumed are mentioned.
Paan masala
According to the Food Safety and Standards Authority of India (FSSAI), Paan masala means the food commonly consumed alone or with paan; common ingredients are lime, areca nut, catechu, coconut, saffron, cardamom, dry fruits with aromatic herbs, and spices with permitted natural colour and non-prohibited flavors; it must not have any other harmful ingredients or coal tar as coloring agent. It must also meet the following criteria: total ash not more than 8% by weight and ash insoluble in dilute hydrochloric acid not more than 0.5% (both on a dry basis).
The paan masala packs shown in Figure 4 are devoid of the original ingredients mentioned in the norms of FSSAI. Out of the eight paan masala packs, three packs were found to have mentioned their ingredients; the labels of ingredients are presented in Figure 2 (local variations) and Figure 4 (packaged paan masala). None of them used original spices and condiments for flavors.
Zarda
Flavored tobacco itself is termed zarda (Figures 3, 4). Both tinned packs and sachets are available readily in any roadside stalls. Bright shiny packs of sachets forming ribbons are a very common site in India. Picture shows sachets of zarda being sold along with paan masala packs.
Betel quid (BQ)
Primary components of a BQ (Figure 3) are the betel leaf, slaked lime, and catechu smeared on it, areca nuts, and optional tobacco in the form of zarda. Readers are directed towards a review by Islam et al. (19) for a more or less extensive reporting of the combinations added in India. In BQ, the variety, flavors, and mixtures added are according to taste, tradition, and regional variations. A BQ (pictures of various preparations with or without tobacco are referred to for the reader) makes it an elaborate process made by paan shop owners. A huge number of combinations and variations of locally made flavoring substances are serially added to the leaf; the leaf is smeared primarily with catechu and lime, then spreading a woody powder (menthol/paan masala/rose-flavored) known as chaman bahar (Figure 3) on the leaf, then adding areca nuts, (Figure 3 depicts components with arrows), condiments like fennel seeds (sugar and colour coated or non-coated), cardamom/clove, saffron, anise seeds, turmeric, coconut (dried/fresh or soaked in saccharine), various sweeteners (brightly colored pieces of vegetables soaked in saccharine) (Figure 3) and flavoring substances, sweetened rose leaf paste (gulkand) (Figure 3), and sweetened flavored paste gulab chatni (gulab refers to rose-flavor) (Figure 3) (12). All these are consumed together in a wrapped betel leaf made by the vendor according to the directions and taste of the consumer. While paan alone with areca nuts and other ingredients without tobacco is considered an independent risk of oral cancer, tobacco/zarda addition increases the risk even further (20). BQ users exhibit BQ-induced lichenoid oral lesions which are seen solely in this user group (21). It resembles lichen planus with some characteristic differences.
Various combinations where areca nuts are consumed are given as follows:
I. Betel leaf + lime + catechu + chaman bahar + with various combinations of sweetened vegetables and condiments (coated and colored), the combination locally called meetha paan, although another nomenclature exists (Figure 3).
II. Betel leaf + lime + zarda + chaman bahar (locally called zarda paan; Figure 3).
III. Betel leaf + areca + lime + fennel seeds + chaman bahar is used in common conception as a mouth freshener (locally called sada paan; sada implies tobacco devoid version).
All other components are used in various combinations and vary widely according to preferences. A few more variations are there in the methods of consumption as well.
Gutka
Gutka is a generic name for a banned product in India (Figure 4). Principally, it is a mixture of artificially flavored (saffron/rose/kewra/paan masala flavors) chewing tobacco and freeze-dried areca nut with menthol, catechu, and lime (Figure 4) (12, 22). Mostly, roasted areca nuts are used to form a dry granular powdery mix packed in sachets (Figure 4). Gutka is mostly banned in India (till May 2013, 26 states banned gutka). Gutka and tobacco industry evaded the gutka ban by selling two separate pouches instead of one sachet. One is labeled as paan masala (the larger one labeled in “A” columns in Figure 4), and the other one is zarda (“B” columns in Figure 4). Gutka is commercially available in different brand names with variations in its composition. Every company has both these consecutive products (kept side by side for reference Figure 4). The numerous chemicals used to make the concoction of gutka are reviewed by Bhisey et al. (23).
Lack of awareness leads to early exposure to the psychoactive agent
Gutka, zarda, and BQ all are separately intended as mouth freshening agents initially introduced in a young population after which it turns out to become an addiction. Chewers begin using areca nuts as a mouth freshener in adolescence, mostly unaware of the damaging effects of oral cancer (24). A cross-sectional study on the prevalence of areca nut uses in the Khasi region of Meghalaya reports a 43.9% use in an age group of 15 years. Almost 30% of respondents in the study were not aware of the ill effects of areca nuts in causing oral cancer (25). As high as 49.5% of the population in India is under oral habits, the cessation of BQ consumption may prevent half of the oral cancers in India (26). Very low concern exists in the Indian psyche about the use of areca nut, areca with paan (BQ called paan), and paan masala (packaged products of commercially produced flavored, mentholated, and saccharinate processed versions of areca nuts) as against oral tobacco uses in forms of zarda and khaini (Figure 5). Through paan masala, BQ, and areca nut use, a hidden and disguised entry takes place for future gutka or oral tobacco use (27). Extensive use of areca nuts in different forms by children, and adolescents, is mostly because of their affordability, pleasant taste, and easy accessibility. In Mumbai, regular consumption of gutka among school students (40%) and college students (70%) is prevalent. About 20% of women and 46.4% of men in Wardha, Maharashtra, consume gutka (8). In this context, it would be worth mentioning that smokeless tobacco is preferred by women because Indian society reprimands smoking and due to this social disapproval, women acquire oral habits of paan masala and subsequently get addicted to gutka. Not only the people from the Indian sub-continent but the immigrants living in the United States and Europe are also habitual users of paan and gutka (28, 29).

Figure 5. (A) Tobacco leaves with the cutting machine; (B) Khaini is made by hand-rubbing finely cut tobacco leaves (C) with a pinch of slaked lime; (D) The prepared khaini is dumped in the cheek pouch.
Areca and its derivative compounds
Areca nuts have a wide variety of chemical compounds including alkaloids, polyphenols, tannins, carbohydrates, fats, proteins, fibre, and various trace elements (30). Alkaloids present mainly are arecoline (principal active ingredient), arecaidine, guvacine, and guvacoline (31, 32). Eugenol or betel oil (a volatile oil) is the key component of the betel leaf. It contains two psychoactive phenols (betel-phenol and chavicol) and cadinene (an alkaloid stimulant) having cocaine-like properties (33).
Arecoline is classified as a Group IIB carcinogenic agent. In the oral cavity, it undergoes nitrosation producing more potent areca-specific N-nitrosamines (22, 34, 35). Four major N-Nitroso compounds, namely, N-Nitrosoguvacoline, N-Nitrosoguvacine, N-(Methylnitrosamino)propionitrile, and N-(Methylnitrosamino)propionaldehyde are produced via nitrosation of arecoline (36, 37).
A few steps towards restriction and banning in India
There had been few interventions specific to BQ use in teenagers, the age group unaware of the dangerous consequences of BQ use and lacking the motivation for cessation (38). Ban on gutka consumption has mostly taken place in India after Supreme Court orders (8). Such bans focus principally on tobacco components specifically and form a warning for tobacco addiction in the zarda pouches but no such warning is present in the paan masala pouches (Figure 4). A very low awareness exists about the carcinogenic effects of areca nuts consumed by the way of paan masala consumption (areca nut is the principal component of paan masala and BQ). Areca nuts and BQ (containing areca) consumed in various ways and combinations fall under Group 1 carcinogen (39). Specifically stated tobacco-devoid-versions of BQ and the paan masalas are carcinogenic by themselves. This carcinogenicity has been the subject of several studies as found in meta-analyses and systematic reviews by Guha et al. (26), Gupta and Johnson (40), Yang et al. (11), and Warnakulasuriya and Chen (41). Areca nut is linked to cancers of the oral cavity, pharynx, larynx, esophagus, gastrointestinal system, and liver.
Areca nut addiction: A neurological perspective
Arecoline, an alkaloid and a potentially addictive component of areca nut, is a cognition enhancer, a psychic stimulant, an anxiolytic, and a sedative (9). Chewing areca nuts brings about positive subjective outcomes, like higher concentration, relaxation, and euphoria. Arecoline has psychoactive effects, as it binds to α4/β2 and α6/β3 subunits of muscarinic and nicotinic acetylcholine receptors (42, 43). Guvacine and arecaidine (other alkaloids present in the areca nut) act as GABA uptake inhibitors and increase the synaptic availability of the neurotransmitter, thereby acting as anxiolytics (9). Arecoline discontinuation results in withdrawal effects with reports of anxiety, mood swings, irritability, and insomnia (44). EEG changes in cortical desynchronization are seen with arecoline exposure (45, 46).
Areca nut related to oral health
‘Gutka Syndrome’, a terminology offered by Chaturvedi (47), aptly described it as a combination of well-known oral disabilities, directly related to gutka consumption. Patients are seen with fibrotic lesions termed OSF in the oral mucus lining. These lesions also affect the muscles of mastication and tongue movements. Gingival recession is seen with enamel loss and razor-sharp discolored teeth (gaps appear between teeth) (48). Reduction in salivary outflow and impediments in speech is noted. The person becomes extremely temperature sensitive, avoids eating spices, and cannot open his mouth fully with typical facial characteristics like loss of facial, buccal fat, and stiffening of soft tissue in the cheek (appearing in chronic users) (49). The inner lining of the cheek appears pale with progressive fibrosis and a kind of stiff mouth-like features develop; lips get inverted with a reduction in the ability to open the mouth (from a loss of gingivobuccal sulcus due to fibrosis) (50). Skinfolds appear in the perioral area, while they try to open their mouth fully, unable to do so. Lower tongue movements and stiffened lips lead to a distinct characteristic in speech, with difficulty in moving the jaw (51, 52). A typical sucking of saliva by a peculiar movement is seen. Fibrosis around the eustachian tube opening leads to hearing loss (47). For khaini and gutka users who dump the contents in the cheek pouch, OSF appears there, whereas in BQ chewers, the fibrosis is located posteriorly in the buccal cavity and extends into the upper and lower sulcus (53). Buccal mucosal cancers are seen frequently in Southeast Asian countries with the habit of chewing BQ.
Severe wear and tear of tooth surface particularly in the incisors and occlusal teeth are noted. Loss of enamel with staining of teeth is noted. Increases in exposed dentin surfaces lead to sensitivity in teeth (54). Root fractures take place from mastication efforts of areca nut chewing. Dentinal surfaces often become sclerosed and such sclerosis may be protective against microbial attack and cavity pain. Areca nuts are reported to be protective against caries (cariostatic) (55, 56). The stained teeth act like a varnish (57). Loss of stagnation areas by a breakdown of tooth edges may lead to lesser observed cavities. Bleeding gums, halitosis, problems in swallowing solid and spicy foods, ulcers on the oral mucosa, and burning sensation of soft tissues are characteristic features of oral health deterioration (48).
Higher salivary secretions may lead to more washing in chewers that inhibits plaque formation; further pH changes due to slaked lime may lead to lower acidity in plaques and thereby lower cavitations (58). Loss of attachment to periodontal tissues with deposition of calculi is seen in areca chewers. Pockets of periodontal tissues were higher in chewers when compared to non-chewers (59). Periodontal-cultured fibroblasts are seen to produce less amount of extracellular matrix proteins indicative of loosened dental attachments.
Brownish red discoloration (Betel chewer’s mucosa) of oral mucosa appears at the sites of placement of the BQ in the buccal cavity (mostly found in older women with chronic and extended uses of BQ) (60). Epithelial hyperplasia with encrustation is seen at the sites where the BQ is placed (58). Microscopic examinations of the area having a wrinkled appearance reveal BQ particles (lime and areca) inside the cells and intracellularly. Oral keratinocytes show a ballooning characteristic. High-risk human papillomavirus subtypes with keratinization and leukoplakia in the mucosa are seen as characteristic features (61). Temporomandibular joint arthrosis and trismus may develop in chronic users (47).
Histopathology of OSF
Hematoxylin and eosin staining is used as a control in various histochemical staining in OSF specimens (Mallory stains are used for identifying stratified layers of keratin; other stains include Masson and Van Gieson stains). Epithelioid alterations in shape, depositions of collagen, infiltration of inflammatory cells, and hyalinization characteristic patterns of fibrillar versus homogenous collagen are routinely used in pathology labs for the detection of OSF grades (62). Lower keratinization, lower number of intercellular bridges, more nuclear and cellular pleomorphism, and more mitotic figures are used as features in subjective histological differentiations in grading squamous cell carcinomas. With higher multinucleated cells and lower intracellular bridges, lower keratinization is associated with higher disease grades (58, 63).
Areca nuts and an analysis of OSF risks
Chewing of areca nut is a major contributing element to OSF development as suggested by epidemiological studies (64, 65). OSF mainly occurs in the buccal mucosa, the mucosal area of the retromolar region, and the soft palate (66, 67). Oral leukoplakia and erythroplakia develop along with OSF.
The clinical features of OSF include taste disorders, dry mouth, dysphagia, changed tone, pain, and restricted opening of mouth and tongue movements. The soft pink oral mucosa hardens and gets whitish, opaque, and tough; the cheeks and the lips get tightly held with fibrotic inelastic bands (palpable from outside) with inabilities to open their mouth (used for staging the disease) (68). Pharynx and esophagus also get fibrotic. The malignant transformation rate is 1.5–15% (69) and the prevalence is high in Southeast Asian countries and India (prevalence of 0.6–6.42) (70).
A recent review by Warnakulasuriya and Chen (41) evaluated the sole implications of areca nut and BQ as an agent for oral carcinoma and estimated the aggregated relative risk (RR) factor for such to be 7.9 (through this meta-meta-analysis for all studies up to 2022). Earlier IARC in 2004 re-evaluated the carcinogenicity of areca nuts to humans with past reports from South Africa (OR 43.9) (71) and Taiwan (OR 13.56). In these countries, BQ preparations are mostly consumed without tobacco and these studies were instrumental in framing the carcinogenic risks associated with areca nut consumption. Before Warnakulasuriya and Chen (41), two systematic reviews and meta-analyses also confirmed the carcinogenicity of BQ without tobacco (40, 72). IARC implicated areca as an independent carcinogen with an RR of 3.22 when consumed alone and an RR of 7.03 when consumed along with tobacco (26, 39).
OSF is considered a precancerous state, a potential condition for the development of oral submucous cancer (73). In a cohort study, it is seen that tobacco users without any precancerous lesions were at a lesser risk of malignant transformation than tobacco consumers with reported OSF (8). The incidence of oral cancer is high in North India due to excessive consumption of gutka in this region (8, 74). The overall harmful effects are oral cancers, cancer of the lip, mouth, tongue, throat, and esophagus, poor oral health, lower immunity, and a range of benign conditions and oral infections, caries, mucositis, gingivitis, sores, and stains. In an Indian scenario, the variety in damage must be categorized for the different forms of areca nut chewers, for example, the only areca nut user-induced OSF or the pan masala-induced OSF, snuff-induced lesions, areca with quid lesions (devoid of tobacco), areca with BQ lesion with tobacco, tobacco-lime (khaini) user’s lesion, gutka chewers mucosa, and likewise. Although the damage looks similar, it shows diversity in clinical and histopathological features (12, 75). In India, while reporting, a mention of the name of a packaged product in the case of paan masala users may be beneficial in categorization. Consumers mostly stay loyal to these brands. The products vary in their quality and labeling (Figure 4). Some of them use synthetic ingredients and flavors, while some have actual formulations of condiments and spices. This may help in the identification of adulterants that should be recorded for inspections and bans. The categorization in research and reporting would definitely narrow down the ambiguity spectrum, focus on the particular characteristics in lesions, and target implications of use.
In a recent review, Cirillo et al. (14) showed that with different BQ components, the risk of development of OSF differed. Chewing of BQ inflorescence increases the risk of OSF and so does the tobacco addiction. The areca leaf alone seemed protective. The studies that calculated the relative risk (RR) of OSF with associations of areca nut chewing were that of Hazare et al. (76) and Ranganathan et al. (77). They calculated the risk of OSF to be most for paan masala chewers and relatively lower values of relative risks were reported for BQ chewers. Mehrotra et al. (78) reported an odds ratio (OR) for OSF to be 3.01 (95% CI: 1.23–7.36) for OSF development in users of paan masala. Both studies significantly reported a positive association of pan masala with the disease. Mehrotra et al. (78) and Khan et al. (79) both reported a higher OR for gutka. Khan et al. (79) reported an OR of 18 + for gutka use to develop OSF with respect to smoking. A total of 33.3% of BQ and gutka chewers presented with tobacco pouch keratosis (79). Maher et al. (80) reported a higher risk with lime and tobacco additions when compared to areca and betel leaf alone. Sari et al. (81) reported that slaked lime increased the damage by higher production of reactive oxygen species (ROS) and they showed that the permeability barrier and damage due to chemical exposure of the alkaloids increase the risk. Lee et al. (82) stated lime induces chromosomal damage with increased mucosal turnover. Lime converts the arecoline and guvacoline into more potent arecaidine and guvacine, increasing further risks (14, 83). Contradicting reports have been seen evaluating whether mature areca or ripe one is more toxic. Sari et al. (84) reported unripe to be more toxic, whilst Jayalakshmi and Mathew (83) reported mature nuts to contain more alkaloids and therefore contribute more towards the development of OSF. Alteration of ingredients changes the property of carcinogenicity and mutagenicity in these mixtures widely (85).
OSF may develop due to a genetic predisposition for damages created by the variety of chemical challenges from any of the pan masala and BQ components. The polymorphic genes mostly implicated to be a factor in OSF development are those that lead to disease pathogenesis.
Molecular mechanism of OSF development
Genes for collagen, matrix metalloproteinases (MMPs) responsible for degradation of heavy metal-activated enzymes, lowering activity of tissue inhibitor of matrix metalloproteinase (TIMP), and transforming growth factor-β (TGF-β) are implicated in the etiology of OSF. Modification of these genes and promoters associated with the expression of transcription is proposed. The polymorphisms in genes making a person more susceptible to OSF are likely to reside in the DNA of these proteins and in a plethora of others proposed in recent studies of proteome, transcriptome, and metagenomic alterations (86). Specific molecular features develop in fibrotic tissues. Epigenetic alterations like hypermethylation in promoters and genes are seen in several genes in OSF patients (87, 88). There is a growing body of evidence that arecoline present in areca nuts is responsible for the development of OSF.
TGF-β and a variety of other pro-inflammatory and profibrotic cytokines are secreted by macrophages and other immune cells recruited by damages created by inflammation. Further ROS-mediated activation and formation of nitroso-amines lead to the induction of various pathways that leads to secretions of numerous cytokines and growth factors (89). Collagen formation by induction of procollagen genes, defective collagen clearance by inhibition of collagenases and metalloproteinases, and stabilization of collagen fibrils by cross-linkages formed by enzymatic activation of cross-linking enzymes are the processes involved with fibrotic changes in the submucosa (62). There is also a deficiency in phagocytosis of crosslinked collagens by fibroblast. Alkaloids in areca nuts stimulate fibroblast cells to produce more collagen. The ratio of collagen chains is altered (90, 91). Induction of genes like Cystatin C, TIMP, and plasminogen activator inhibitor (PAI) by TGF-β leads to the inhibition of collagen degradation (92, 93). Arecoline elevates proinflammatory and profibrotic cytokines and growth factors, in turn, promotes collagen synthesis. Proteinases like MMP- 9 and MMP-2 are inhibited by the areca alkaloids to create a disequilibrium in ECM dynamics (65, 94). A higher concentration of copper in areca nut leads to increased salivary and serum copper concentrations. Lysyl oxidase, an enzyme that cross-links collagen and elastin proteins, is induced by copper. Increased cross-linked collagen in fibrotic tissues renders them resistant to collagenases and thereby phagocytosis is inhibited (65, 93–98).
OSF development in betel nut users is due to the presence of arecoline, the major alkaloid present in areca nuts (99, 100). Approximately, 7.6% of OSF patients develop oral cancer in India (101). The promotion stage from a premalignant lesion, OSF to a malignant form, oral squamous cell carcinoma (OSCC) is a lengthy and irreversible process. Once OSF is diagnosed and the patient seeks medical help interventions and cessation of habit lead to interruption in the process of carcinogenesis and malignancy. This leads to a decrease in the malignant transformation rate of OSF (Figure 6).
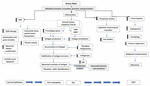
Figure 6. Mechanism of development of OSF and transformation to OSCC. This flowchart is made based on the data compiled in reviews of (62, 70, 102).
Conclusion
The traditional uses of BQ called the paan supari include uses in hospitality, religious offerings, depiction as a social and ethnic symbol and as a medicinal ingredient makes the task of controlling and restriction of its use a daunting one in India. Aggressive commercialization, economic benefits through cultivation, lack of education, and awareness in India had turned this paan masala addiction into a situation where drastic steps are needed to be taken by the government. In a country of 1.3 billion, a huge heterogeneity in demography and a predominance in oral habits due to its cultural identity will be a challenge to target populations and devise selective restrictions for paan masala (an item recognized as food in Indian law). Imposing selective bans and restrictions on a huge number of adulterations, sold under the name of paan masala, are necessary. Initiatives for mandatory categorization of items, declaration of their contents, and level of use of ingredients will further elucidate the toxicity that is hidden and sold under the name of an oral freshening and aromatic agent.
Author contributions
The manuscript is solely and fully conceived, developed, interpreted, analyzed, and drafted by SB. All pictures are taken by SB. SB is accountable for the content of the work.
Funding
No grant was received from any funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank all the scientists and working groups for their effort to elucidate the harmful effects of areca nut in betel quid and paan masala preparation.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. International Agency for Research on Cancer. Global Cancer Observatory. (2019). Available online at: https://gco.iarc.fr/databases.php (accessed September 20, 2022).
2. Ren Z, Hu C, He H, Li Y, Lyu J. Global and regional burdens of oral cancer from 1990 to 2017: results from the global burden of disease study. Cancer Commun. (2020) 40:81–92. doi: 10.1002/cac2.12009
3. Singh P, Yadav A, Singh L, Mazumdar S, Sinha D, Straif K, et al. Areca nut consumption with and without tobacco among the adult population: a nationally representative study from India. BMJ Open. (2021) 11:e043987.
4. Moss W. The seeds of ignorance — consequences of a booming betel-nut economy. N Engl J Med. (2022) 387:12. doi: 10.1056/NEJMp2203571
5. Ahuja S, Ahuja U. Betel leaf and betel nut in India: History and uses. Asian Agrihist. (2011) 15:13–35.
6. Global Adult Tobacco Survey. Second Round Tata Institute of Social Sciences (TISS), Mumbai and Ministry of Health and Family Welfare, Government of India. (2016-2017).
7. Little M, Papke R. Betel, the orphan addiction. J Addict Res Ther. (2015) 6:3. doi: 10.4172/2155-6105.1000e130
8. Niaz K, Maqbool F, Khan F, Bahadar H, Hassan FI, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health. (2017) 9:e2017009. doi: 10.4178/epih.e2017009
9. Osborne PG, Ko YC, Wu MT, Lee C. Intoxication and substance use disorder to Areca catechu nut containing betel quid: A review of epidemiological evidence, pharmacological basis and social factors influencing quitting strategies. Drug Alcohol Depend. (2017) 79:187–97. doi: 10.1016/j.drugalcdep.2017.06.039
10. Winstock A. Areca nut-abuse liability, dependence and public health. Addict Biol. (2002) 7:133–8.
11. Yang J, Wang Z, Huang L, Yu T, Wan S, Song J, et al. Do betel quid and areca nut chewing deteriorate prognosis of oral cancer? A systematic review, meta-analysis, and research agenda. Oral Dis. (2021) 27:1366–75. doi: 10.1111/odi.13456
12. Patidar KA, Parwani R, Wanjari SP, Patidar AP. Various terminologies associated with areca nut and tobacco chewing: a review. J Oral Maxillofac Pathol. (2015) 19:69–76.
13. Ko YC, Huang YL, Lee CH, Chen MJ, Lin LM, Tsai CC. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J Oral Pathol Med. (1995) 24:450–3.
14. Cirillo N, Duong P, Er W, Do C, De Silva M, Dong Y, et al. Are there betel quid mixtures less harmful than others? A scoping review of the association between different betel quid ingredients and the risk of oral submucous fibrosis. Biomolecules. (2022) 12:664. doi: 10.3390/biom12050664
15. Johnston GA, Krogsgaard-Larsen P, Stephanson A. Betel nut constituents as inhibitors of gamma-aminobutyric acid uptake. Nature. (1975) 258:627–8.
16. Kadi AA, Attwa MW, Rahman AF. A preliminary study of arecoline and guvacoline presence in the saliva of a “betel-quid” chewer using liquid-chromatography ion trap mass spectrometry. Eur J Mass Spectrom. (2013) 19:391–7.
17. Liu Y, Lee M, Chen C, Lin Y, Ho H. Purification of Cu/Zn superoxide dismutase from piper betel leaf and its characterization in the oral cavity. J Agric Food Chem. (2015) 63:2225–32.
18. Nair UJ, Obe G, Friesen M, Goldberg MT, Bartsch H. Role of lime in the generation of reactive oxygen species from betel-quid ingredients. Environ Health Perspect. (1992) 98:203–5.
19. Islam S, Muthumala M, Matsuoka H, Uehara O, Kuramitsu Y, Chiba I, et al. How each component of betel quid is involved in oral carcinogenesis: mutual interactions and synergistic effects with other carcinogens—a review article. Curr Oncol Rep. (2019) 21:53.
20. Merchant A, Husain SS, Hosain M, Fikree FF, Pitiphat W, Siddiqui AR, et al. Paan without tobacco: an independent risk factor for oral cancer. Int J Cancer. (2000) 86:128–31.
21. Arya S, Vengal M, Raju B, Patil N, Sathosker S, Bateja S, et al. Betel quid oral lichenoid lesions: a hospital based cross-sectional study. J Investig Clin Dent. (2017) 8. doi: 10.1111/jicd.12180
22. Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. (2004) 19:251–62.
23. Bhisey RA, Boucher BJ, Chen TH, Gajalakshmi V, Gupta PC, Hecht SS, et al. In: IARC working group on the evaluation of carcinogenic risk to Humans editor. Betel-quid and Areca-Nut Chewing and Some Areca-nut-Derived Nitrosamines. Lyon: IARC Press (2004). 2004 p.
24. Moss J, Kawamoto C, Pokhrel P, Paulino Y, Herzog T. Developing a betel quid cessation program on the island of Guam. Pac Asia Inq. (2015) 6:144–50.
25. Snigdha S, Bajwa T, Anand S, Mohan L, Goyal K, Mittal M, et al. Cross-sectional study on prevalence of betel nut chewing among the youth of Meghalaya, North East Region of India: development of multifaceted prevention strategy. Asian Pac J Health Sci. (2021) 8:185–90.
26. Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer. (2014) 135:1433–43.
27. Chandra PS, Mulla U. Areca nut: the hidden Indian gateway to future tobacco use and oral cancers among youth. Indian J Med Sci. (2007) 61:319–21.
28. Gupta PC, Ray CS. Epidemiology of betel quid usage. Ann Acad Med Singap. (2004) 33(4 Suppl.):31–6.
29. Nelson BS, Heischober B. Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med. (1999) 34:238–43.
30. Lord GA, Lim CK, Warnakulasuriya S, Peters TJ. Chemical and analytical aspects of areca nut. Addict Biol. (2002) 7:99–102. doi: 10.1080/13556210120091455
31. Huang JL, McLeish MJ. High-performance liquid chromatographic determination of the alkaloids in betel nut. J Chromatogr. (1989) 475:447–50.
32. Chen X, He Y, Deng Y. Chemical composition, pharmacological, and toxicological effects of betel nut. Evid Based Complement Alternat Med. (2021) 18:1808081. doi: 10.1155/2021/1808081
33. Pickwell SM, Schimelpfening S, Palinkas LA. ‘Betelmania’ betel quid chewing by Combodian women in the United States and its potential health effects. West J Med. (1994) 160:326–30.
34. Volgin A, Bashirzade A, Amstislavskaya T, Yakovlev O, Demin K, Ho Y, et al. DARK classics in chemical neuroscience: arecoline. ACS Chem Neurosci. (2019) 10:2176–85.
35. Nair J, Nair UJ, Ohshima H, Bhide SV, Bartsch H. Endogenous nitrosation in the oral cavity of chewers while chewing betel quid with or without tobacco. IARC Sci Publ. (1987) 84:465–9.
36. IARC. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans Tobacco Habits Other Than Smoking; Betel quid and Areca-nut Chewing; and Some Related nitrosamines. (Vol. 37). Lyon: IARC (1985). 263.
37. IARC. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Betel-Quid and Areca-Nut Chewing and Some Areca-Nut-Derived Nitrosamines. (Vol. 85). Lyon: IARC (2004).
38. Chen G, Hsieh M, Chen A, Kao N, Chen M. The effectiveness of school educating program for betel quid chewing: a pilot study in Papua New Guinea. J Chin Med Assoc. (2018) 81:352–7. doi: 10.1016/j.jcma.2017.10.001
39. IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Personal Habits and Indoor Combustions. A Review of Human Carcinogens. Lyon: IARC (2012).
40. Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLoS One. (2014) 9:e113385. doi: 10.1371/journal.pone.0113385
41. Warnakulasuriya S, Chen THH. Areca nut and oral cancer: evidence from studies conducted in humans. J Dent Res. (2022) 101:1139–46. doi: 10.1177/00220345221092751
42. Papke RL, Horenstein NA, Stokes C. Nicotinic activity of arecoline, the psychoactive element of “betel nuts”, suggests a basis for habitual use and anti-inflammatory activity. PLoS One. (2015) 10:e0140907. doi: 10.1371/journal.pone.0140907
43. Papke RL, Hatsukami DK, Herzog TA. Betel quid, health, and addiction. Subst Use Misuse. (2020) 55:1528–32. doi: 10.1080/10826084.2019.1666147
44. Giri S, Idle JR, Chen C, Zabriskie TM, Krausz KW, Gonzalez FJ. A metabolomic approach to the metabolism of the areca nut alkaloids arecoline and arecaidine in the mouse. Chem Res Toxicol. (2006) 19:818–27.
45. Chu NS. Effects of betel chewing on the central and autonomic nervous systems. J Biomed Sci. (2001) 8:229–36.
46. Osborne PG, Chou TS, Shen TW. Characterization of the psychological, physiological and EEG profile of acute betel quid intoxication in naı–ve subjects. PLoS One. (2011) 6:e23874. doi: 10.1371/journal.pone.0023874
48. Parmar G, Sangwan P, Vashi P, Kulkarni P, Kumar S. Effect of chewing a mixture of areca nut and tobacco on periodontal tissues and oral hygiene status. J Oral Sci. (2008) 50:57–62.
49. Anand R, Dhingra C, Prasad S, Menon I. Betel nut chewing and its deleterious effects on oral cavity. J Cancer Res Ther. (2014) 10:499–505.
50. Pindborg JJ, Sirsat SM. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. (1966) 22:764–79.
51. Shirzaii M, Momeni Z. Evaluation of oral submucosal fibrosis and related factors in areca nut users. Zahedan J Res Med Sci. (2013) 15:38–42.
52. Vijayakumar M, Priya D. Physiotherapy for improving mouth opening & tongue protrusion in patients with oral submucous fibrosis (OSMF) – case series. Int J Pharm Sci Health Care. (2013) 3:50–8.
53. Kumar V, Sindhu VA, Rathanaswamy S, Jain J, Pogal JR, Akhtar N, et al. Cancers of upper gingivobuccal sulcus, hard palate and maxilla: a tertiary care centre study in North India. Natl J Maxillofac Surg. (2013) 4:202–5. doi: 10.4103/0975-5950.127652
54. Yeh CJ. Fatigue root fracture: a spontaneous root fracture in non-endodontically treated teeth. Br Dent J. (1997) 182:261–6.
56. de Miranda CM, van Wyk CW, van der Biji P, Basson NJ. The effect of areca nut on salivary and selected organisms. Int Dent J. (1996) 46:350–6.
58. Trivedy CR, Craig G, Warnakulasuriya S. The oral health consequences of chewing areca nut. Addict Biol. (2002) 7:115–25.
59. Chatrchaiwiwatana S. Dental caries and periodontitis associated with betel quid chewing: analysis of two data sets. J Med Assoc Thai. (2006) 89:1004–11.
60. Reichart PA, Schmidtberg W, Scheifele CH. Betel chewer’s mucosa in elderly Cambodian women. J Oral Pathol Med. (1996) 25:367–70.
61. Maeda H, Ikeda N, Zain R, et al. Detection of human papillomaviruses in betel chewer’s mucose and leukoplakias of Cambodian patients. In: Proceedings of the 2nd Asian Pacific Workshop for Oral Mucosal Lesions. Chiang Mai (1995).
62. Shih Y, Wang T, Shieh T, Tseng Y. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci. (2019) 20:2940. doi: 10.3390/ijms20122940
63. Ray JG, Ranganathan K, Chattopadhyay A. Malignant transformation of oral submucous fibrosis: overview of histopathological aspects. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:200–9.
64. Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. (2006) 42:561–8.
65. Angadi PV, Rao SS. Areca nut in pathogenesis of oral submucous fibrosis: revisited. Oral Maxillofac Surg. (2011) 15:1–9.
66. Dionne KR, Warnakulasuriya S, Zain RB, Cheong SC. Potentially malignant disorders of the oral cavity: current practice and future directions in the clinic and laboratory. Int J Cancer. (2015) 136:503–15.
67. Chole R, Gondivkar S, Gadbail A, Balsaraf S, Chaudhary S, Dhore S, et al. Review of drug treatment of oral submucous fibrosis. Oral Oncol. (2012) 48:393–8.
68. Tom A, Baghirath V, Krishna B, Ganepalli A, Kumar JV, Mohan SP. Ultrastructural changes of collagen in different histopathological grades of oral submucous fibrosis. J Pharm Bioallied Sci. (2019) 11:S309–13.
69. Wang Y, Tail Y, Wang W, Chen C, Kao Y, Chen Y, et al. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health. (2014) 14:99. doi: 10.1186/1472-6831-14-99
70. Shen Y, Shih Y, Fuh L, Shieh T. Oral submucous fibrosis: A review on biomarkers, pathogenic mechanisms, and treatments. Int J Mol Sci. (2020) 21:7231. doi: 10.3390/ijms21197231
71. van Wyk CW, Stander I, Padayachee A, Grobler-Rabie AF. The areca nut chewing habit and oral squamous cell carcinoma in South African Indians. A retrospective study. S Afr Med J. (1993) 83:425–9.
72. Petti S, Masood M, Scully C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in south-east Asia. A meta-analysis of observational studies. PLoS One. (2013) 8:e78999. doi: 10.1371/journal.pone.0078999
73. Gunjal S, Pateel DG, Yang Y, Doss JG, Bilal S, Maling TH, et al. An overview on betel quid and areca nut practice and control in selected Asian and south east Asian countries. Subst Use Misuse. (2020) 55:1533–44. doi: 10.1080/10826084.2019.1657149
74. Mehrotra R, Singh M, Gupta RK, Singh M, Kapoor AK. Trends of prevalence and pathological spectrum of head and neck cancers in North India. Indian J Cancer. (2005) 42:89–93.
75. Zain RB, Ikeda N, Gupta PC, Warnakulasuriya S, Wyk CW, Shrestha P, et al. Oral mucosal lesions associated with betel quid, arecanut and tobacco chewing habits: Oral Pathol. Med. J. Kuala Lumpur, Malaysia, November 25-27, 1996. J Oral Pathol Med. (1996) 28:1–4.
76. Hazare VK, Goel RR, Gupta PC. Oral submucous fibrosis, areca nut and pan masala use: a case-control study. Natl Med J India. (1998) 11:299.
77. Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: a case-control study in Chennai. South India J Oral Pathol Med. (2004) 33:274–7. doi: 10.1111/j.0904-2512.2004.00116.x
78. Mehrotra D, Kumar S, Agarwal GG, Asthana A, Kumar S. Odds ratio of risk factors for oral submucous fibrosis in a case control model. Br J Oral Maxillofac Surg. (2013) 51:e169–73.
79. Khan A, Ongole R, Baptist J, Srikant N, Lukmani F. Patterns of tobacco use and its relation to oral precancers and cancers among individuals visiting a tertiary hospital in south India. J Contemp Dent Pract. (2020) 21:304–9.
80. Maher R, Lee AJ, Warnakulasuriya KA, Lewis JA, Johnson NW. Role of areca nut in the causation of oral submucous fibrosis: a case-control study in Pakistan. J Oral Pathol Med. (1994) 23:65–9.
81. Sari EF, Prayogo GP, Loo YT, Zhang P, McCullough MJ, Cirillo N. Distinct phenolic, alkaloid and antioxidant profile in betel quids from four regions of Indonesia. Sci Rep. (2020) 10:16254.
82. Lee Y, Yang L, Hu F, Peng C, Yu C, Yu C. Elevation of twist expression by arecoline contributes to the pathogenesis of oral submucous fibrosis. J Formos Med Assoc. (2016) 115:311–7.
83. Jayalakshmi A, Mathew A. Chemical composition and processing. In: Bavappa K, Nair M, Kumar T editors. The Arecanut Palm. Kerala: Central Plantation Crops Research Institute (1982). p. 225–44.
84. Sari EF, McCullough M, Cirillo N. Oral pre-malignant and malignant lesion detection among Indonesians; the prevalence and risk factors. Head Neck. (2017) 39 (Suppl. 1):E58.
85. Hazarey VK, Erlewad DM, Mundhe KA, Ughade SN. Oral submucous fibrosis: Study of 1000 cases from central India. J Oral Pathol Med. (2007) 36:12–7.
86. Hernandez BY, Zhu X, Goodman MT, Gatewood R, Mendiola P, Quinata K, et al. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. (2017) 12:e0172196. doi: 10.1371/journal.pone.0172196
87. Huang S, Lee H, Mar K, Ji D, Huang M, Hsia K. Loss expression of O6-methylguanine DNA methyltransferase by promoter hypermethylation and its relationship to betel quid chewing in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 109:883–9.
88. Islam S, Uehara O, Matsuoka H, Kuramitsu Y, Adhikari BR, Hiraki D, et al. DNA hypermethylation of sirtuin 1 (SIRT1) caused by betel quid chewing—a possible predictive biomarker for malignant transformation. Clin Epigenet. (2020) 12:12.
89. Chang Y, Muthukumaran RB, Chen J, Chang H, Hung Y, Hu C, et al. Simultaneous determination of areca nut-and tobacco-specific alkaloids in saliva by LC-MS/MS: distribution and transformation of alkaloids in oral cavity. J Hazard Mater. (2022) 426:128116.
90. Liu C, Liao Y, Hsieh P, Yu C, Chueh PJ, Lin T, et al. miR-1246 as a therapeutic target in oral submucosa fibrosis pathogenesis. J Formos Med Assoc. (2019) 118:1093–8.
91. Kuo MY, Chen HM, Hahn LJ, Hsieh CC, Chiang CP. Collagen biosynthesis in human oral submucous fibrosis fibroblast cultures. J Dent Res. (1995) 74:1783–8.
92. Aziz SR. Oral submucous fibrosis: Case report and review of diagnosis and treatment. J Oral Maxillofac Surg. (2008) 66:2386–9.
93. Arakeri G, Brennan PA. Oral submucous fibrosis: an overview of the aetiology, pathogenesis, classification, and principles of management. Br J Oral Maxillofac Surg. (2013) 51:587–93.
94. Shieh DH, Chiang LC, Shieh TY. Augmented mRNA expression of tissue inhibitor of metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and safrole as a possible pathogenesis for oral submucous fibrosis. Oral Oncol. (2003) 39:728–35.
95. Shieh D, Chiang L, Lee C, Yang Y, Shieh T. Effects of arecoline, safrole, and nicotine on collagen phagocytosis by human buccal mucosal fibroblasts as a possible mechanism for oral submucous fibrosis in Taiwan. J Oral Pathol Med. (2004) 33:581–7.
96. Sachdev P, Freeland-Graves J, Beretvas S, Sanjeevi N. Zinc, copper, and iron in oral submucous fibrosis: a meta-analysis. Int J Dent. (2018) 2018:3472087.
97. Vallet SD, Ricard-Blum S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays Biochem. (2019) 63:349–64.
98. Raja KB, Hazarey VK, Peters TJ, Warnakulasuriya S. Effect of areca nut on salivary copper concentration in chronic chewers. Biometals. (2007) 20:43–7.
99. Hsieh Y, Wu K, Chen H, Deng Y. Arecoline activates latent transforming growth factor beta1 via mitochondrial reactive oxygen species in buccal fibroblasts: suppression by epigallocatechin-3-gallate. J Formos Med Assoc. (2018) 117:527–34.
100. World Health Organization. Review of Areca (Betel) Nut and Tobacco Use in the Pacific: A Technical Report. Geneva: World Health Organization (2012).
102. Das A, Orlan E, Duncan K, Thomas H, Ndumele A, Ilbawi A, et al. Areca nut and betel quid control interventions: halting the epidemic. Subst Use Misuse. (2020) 55:1552–9. doi: 10.1080/10826084.2019.1686022