1. Introduction
A multisystem involving autoimmune inflammatory disease is called Vogt-Koyanagi-Harada disease, characterized by panuveitis with neuro-cutaneous features (1). The recurrent phase of the anterior uveitis is the most common period for developing glaucoma secondary to VKH due to posterior synechiae and pupillary block, peripheral anterior synechiae, trabeculitis, clogging of the trabecular meshwork by inflammatory cells, and the prolonged use of glucocorticoids (2).
Aurolab Aqueous Drainage Implant (AADI) is a Baerveldt type, valveless glaucoma drainage device (GDD), which shunts aqueous humor from the anterior or posterior chamber to an episcleral plate in the equatorial region through a tube. GDDs are frequently utilized to decrease intraocular pressure (IOP) in refractory glaucoma patients who do not respond to penetrating glaucoma procedures or the most aggressive medication treatment (3, 4).
Endophthalmitis is an uncommon sight-threatening complication following GDD implant, and the precise incidence is unknown (4). Delayed endophthalmitis after GDD was not reported until 2 years after surgery. After GDD implantation, late-onset endophthalmitis has been documented up to 2 years after surgery (5). Tube exposure is classified as a significant risk factor in these cases (4). In contrast, there was no consensus for isolated panophthalmitis other than panophthalmitis with orbital cellulitis after GDD surgery (6).
This is a rare case of panophthalmitis after AADI surgery, which was managed with evisceration and complete removal of the device including tube and plate.
2. Case report
A male aged 26 years presented with sudden visual loss, moderate pain, redness, and purulent discharge in the right eye for 6 days. He underwent an AADI on his right eye 2 months ago for refractory glaucoma due to VKH. He had no systemic features of VKH. He was followed up regularly, maintaining 6/60 visual acuity in his right eye and no light perception (NPL) in his left eye, and good-controlled IOP in the range of 10–12 mmHg without antiglaucoma medications in either eye.
Examination revealed that the vision in his right eye had deteriorated to NPL, with a slightly swollen eyelid and matted eyelashes. Slit-lamp examination showed severe conjunctival and ciliary congestion, a total hypopyon with the melted cornea, an exposed corneal stitch at around 11 O’clock, and an AADI plate in the superotemporal quadrant, and the scleral patch graft was used to cover the tube (Figures 1, 2).
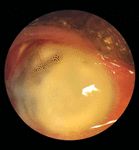
Figure 1. Right eye showing severe conjunctival and ciliary congestion, a total hypopyon with the melted cornea, and an exposed corneal stitch at around 11 o’clock.

Figure 2. Right eye showing AADI plate, scleral patch graft, exposed suture with the melted cornea, and conjunctival and ciliary congestion.
There was no conjunctival or ciliary congestion with mild corneal edema, ectropion uvae, peripheral anterior synechiae, and complicated cataract with no posterior segment view of the left eye (Figure 3).

Figure 3. Left eye showing mild corneal edema, ectropion uvae, peripheral anterior synechiae, and complicated cataract.
B-scan showed moderate to dense vitreous opacities that persist in low gain as well (Figure 4). A complete blood count showed neutrophilic leukocytosis.
Panophthalmitis of the right eye was the patient’s diagnosis. He was immediately hospitalized and treated with oral ciprofloxacin 400 mg four times daily, topical antibiotics including moxifloxacin 0.5%, fortified ceftazidime 50 mg/ml, and vancomycin 25 mg/ml every 30 min, atropine sulfate 1%, and topical and oral prednisolone.
Evisceration was later performed, and the AADI, including tube and plate, and suture were completely removed and sent for microbiological and cytological analysis.
There was a purulent discharge-filled tube and plate while removed. Microbiological results showed no growth for bacteria and fungus. The cytological report showed an acute inflammatory reaction.
3. Discussion
Post-operative infection in patients treated with GDD is rare. Endophthalmitis rates were 0.8 and 2%, respectively, in two long-term trials evaluating outcomes after utilizing the Ahmed™ and Baerveldt™ glaucoma devices, without any major difference between implants (7, 8).
Late-onset endophthalmitis is mostly related to GDD implants, and tube erosion appears to be a major risk factor (3, 5, 9). Failure to follow post-surgical care recommendations, such as avoiding eye rubbing and non-compliance with topical medications, may raise the chance of early infection.
Organisms that cause GDD-related endophthalmitis in adults include coagulase-negative and coagulase-positive Staphylococcus species, Streptococcus pneumonia, and Pseudomonas aeruginosa (4, 10, 11). Pseudomonas aeruginosa causes severe endophthalmitis, which can initially be treated by evisceration (3).
Recommendations for removal of GDD in case of endophthalmitis are unclear (3, 12).
Our case was presented as panophthalmitis 2 months after AADI surgery, with no evidence of tube erosion or footplate exposure. He was hospitalized and started treatment immediately. After evisceration and complete removal of the AADI device and suture, the microbiological analysis showed negative results for bacterial and fungal growth. The cytological analysis showed an inflammatory reaction, acute in nature, and blood analysis showed neutrophilic leukocytosis.
We believe that the two most likely explanations for this instance are either an immunological reaction by VKH disease or infection by unusual microorganisms that cannot be detected with standard culture media and staining procedures. Due to the lack of related conditions published in the journals, the major precipitating event and/or cause are unclear (to the best of our knowledge).
4. Conclusion
Endophthalmitis is an uncommon and typically delayed consequence following GDD implantation. GDD-associated endophthalmitis can be fatal and requires immediate treatment. In the case of panophthalmitis, this may be due to a comparatively long delay between symptom onset and presentation.
Acknowledgments
The authors thank Vitreo-retina, and Oculoplasty and Ocular Oncology Departments of Ispahani Islamia Eye Institute and Hospital, Dhaka, Bangladesh.
Declaration of patient consent
The authors attest that the proper patient permission form has been acquired. While reasonable attempts will be taken to keep the patient’s identity hidden, perfect anonymity cannot be guaranteed. The patient has given his approval in the form for his photos and other clinical information to be reported in the journal without his name attached.
References
1. Street D, Sivaguru A, Sreekantam S, Mollan S. Vogt-koyanagi-harada disease. Pract Neurol. (2019) 19:364–7. doi: 10.1136/practneurol-2018-002152
2. Wada S. Ultrasound biomicroscopic study of ciliary body changes in the post-treatment phase of vogt-koyanagi-harada disease. Br J Ophthalmol. (2002) 86:1374–9. doi: 10.1136/bjo.86.12.1374
3. Al-Torbak A, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward D. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. (2005) 89:454–8.
4. Gedde S, Scott I, Tabandeh H, Luu K, Budenz D, Greenfield D, et al. Late endophthalmitis associated with glaucoma drainage implants. Ophthalmology. (2001) 108:1323–7.
5. Gedde S, Herndon L, Brandt J, Budenz D, Feuer W, Schiffman J. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. (2012) 153:804–14.e1. doi: 10.1016/j.ajo.2011.10.024
6. Esporcatte B, Teixeira L, Rolim-de-Moura C. Panophthalmitis with orbital cellulitis following glaucoma drainage implant surgery in a pediatric patient. Arquivos Brasileiros de Oftalmol. (2016) 79:20160037. doi: 10.5935/0004-2749.20160037
7. Christakis P, Kalenak J, Tsai J, Zurakowski D, Kammer J, Harasymowycz P, et al. The ahmed versus baerveldt study. Ophthalmology. (2016) 123:2093–102. doi: 10.1016/j.ophtha.2016.06.035
8. Barton K, Feuer W, Budenz D, Schiffman J, Costa V, Godfrey D, et al. Three-year treatment outcomes in the ahmed baerveldt comparison study. Ophthalmology. (2014) 121:1547–57.e1. doi: 10.1016/j.ophtha.2014.01.036
9. AlHadlaq A, AlMalki S, AlShahwan S. Late onset endophthalmitis associated with unexposed glaucoma valved drainage device. Saudi J Ophthalmol. (2016) 30:125–7. doi: 10.1016/j.sjopt.2015.12.005
10. Law S. Retinal complications after aqueous shunt surgical procedures for glaucoma. Arch Ophthalmol. (1996) 114:1473. doi: 10.1001/archopht.1996.01100140671004
11. Perkins T. Endophthalmitis after placement of a molteno implant. Ophthalmic Surgery Lasers Imaging Retina. (1990) 21:733–4. doi: 10.3928/1542-8877-19901001-15
