Introduction
Inflammatory in the central nervous system are mediated by microglia, which are natural immune system cells. According to studies on the central nervous system, microglia have been linked to neurodegenerative illnesses such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and Creutzfeldt–Jakob disease, among others (1, 2). The emergence of neuronal, glial-microglial, and immunological responses similar to those of the central nervous system in the retina and optic nerve tissues has made neuroprotection, neuroinflammation, and immunomodulation studies in ophthalmologic diseases a current issue. The loss of retinal ganglion cells (RGCs) is a hallmark of the multifactorial neurodegenerative illness of the optic nerve known as glaucoma. It has been demonstrated that RGC loss, degeneration of axons and somas of the cells, increased gliosis, and microglial cell activation are important in glaucoma progression.
Although the leading risk factor for glaucomatous optic nerve damage is high intraocular pressure (IOP), there is damage in some people even though IOP is within normal limits. In contrast, there are individuals who do not develop optic neuropathy with high IOP (3, 4). This situation suggests that glaucomatous pathophysiology is associated with multiple mechanisms. The main goal of glaucoma treatment is to protect RGCs. Besides reducing IOP, new treatment strategies aim to develop molecules that prevent loss of RGC by neuroprotective effects and agents that reduce neuroinflammation by suppressing gliosis/microglial activation (5–8).
Neuroinflammation can be defined as any immune-related response generated by various cell types, including astrocytes, microglia, and peripheral cells that occur in the optic nerve head or retina (9). Microglia cells are the primary immune system cells in the central nervous system. Although microglial cells are very important in maintaining homeostasis, they have been shown to be the cause of many neurodegenerative diseases that disrupt the balance of microsystems. Microglial cells are activated under stress conditions by interacting with damaged cells in situations that pose a threat to neuronal survival (5, 8, 10). Iba-1 is a microglia-specific marker and used to characterize microglial morphology, distribution, and numbers.
This study aimed to investigate the numerical and morphological effects of brimonidine, which is a molecule with a clinical neuroprotective effect, on microglia cells in the neuroinflammatory process by creating a transient retinal ischemia model in rats.
Research elaborations
Selection of experimental animals and forming the groups
The study was developed in accordance with the ARVO position on the use of experimental animals in ophthalmology and vision research, and it received approval from the medical faculty’s Ethics Committee. A model of transient retinal ischemia was developed in the right eyes of male Wistar rats (250–300 gr). Rats with mechanical optic nerve damage were divided into three groups: eyes treated with topical brimonidine (group 1, n = 4), eyes treated with sham drops (artificial tears) (group 2, n = 4), and eyes not operated as a control group (group 3, n = 4). Treatment was applied topically. An enucleation procedure was performed on treated rats under xylazine-ketamine anesthesia at the end of 4 weeks, and the rats were sacrificed.
Creation of transient retinal ischemia model
In addition to xylazine-ketamine anesthesia, 0.5% topical proparacaine HCl was applied to the rats. Further, 1% cyclopentolate and 0.5% tropicamide were used for pupil dilatation. Under the operating microscope, the anterior chamber of the right eye was cannulated with a 30 gauge needle attached to a 1 L of balanced saline solution (BSS; Alcon Laboratories, Fort Worth, TX, USA). IOP was increased to 70–80 mmHg for 50 min. Pressure-related ischemia was confirmed by examining in terms of whitening of the eyes and arterial pulse deficiency with the ophthalmoscope. After the procedure, the ocular fundus was examined for confirmation of reperfusion. An antibiotic ointment was applied and the operation was ended.
Pre-paration of tissues
-Enucleated eyes were fixated with 10% buffered formalin. Alcohol was added to the fixation solution, and an intravitreal alcohol injection was conducted. The eyes are separated into two pieces from anterior to posterior. For paraffin blocks, hematoxylin–eosin staining (H & E) was used for examination.
A pathologist, who was experienced in this subject and was not informed about pre-parations, sampled microglia in tissue samples from 10 visual areas using X40 visual magnification, starting at the back of each retina.
For immunohistochemical investigations, Iba-1 (1:1000, clone: polyclonal, EDTA, Synaptic Systems, Göttingen, Germany) was used.
Statistical analysis
For the purpose of determining statistical significance, the Kruskal–Wallis and Friedman tests were used. SPSS version 18.0 for Windows was used for the statistical analysis (SPSS Inc., Chicago, IL, USA). The threshold for statistical significance was set at p < 0.05.
Morphological analysis of microglial phenotypes
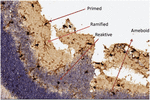
The counting and evaluation of Iba-1 immunoreactive microglia cells in all retinal layers were performed. The four distinct morphological phenotypes described in previous studies were in an easily recognizable state in the retinal layers (1, 11–13). According to classically different activation states, microglia phenotypes were identified as ramified, primed, reactive, and amoeboid-phagocytic (Figure 1).
Ramified microglia are cells that possess a small cell body and highly branched extensions. Primed microglia are cells that have more ellipsoid cell bodies in comparison to ramified cells, and their branchings are not as long and as prominent as ramified cells (Figure 2).

Figure 2. Ramified microglia, their small bodies and highly branched extensions, primed microglia, their larger cell bodies and branched extensions similar to ramified cells.
Amoeboid-shaped cell bodies can be found in both reactive and amoeboid microglia. Reactive microglia cells typically have extensions with branchings that extend beyond the cell body width (Figure 3A). Amoeboid microglia are cells that do not have extensions or that have unbranched extensions that appear to be along the cell body diameter (Figure 3B).

Figure 3. (A) Reactive microglia, amoeboid cell bodies, a small number of extensions and branchings and (B) Ameboid microglia, extensions growing from the cell body and not having branchings.
Results
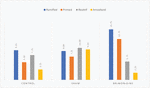
In the transient retinal ischemia model, the number of Iba-1 positive microglia cells was 43.3 ± 6.02 in the control group, 100.67 ± 7.50 in the sham treatment group, and 57.67 ± 14.64 in the topical brimonidine group. The decrease in the total number of Iba-1 positive microglial cells in the topical brimonidine group was statistically significant (p < 0.05).
There was no statistically significant difference between the groups when we compared ramified and primed microglia cells (Chart 1).
The number of reactive microglial cells was 10 ± 1.41 in the sham treatment group and 5.75 ± 1.71 in the topical brimonidine group (p < 0.05). The number of amoeboid-phagocytic microglial cells was 9.5 ± 1.29 in the sham treatment group and 2.25 ± 0.50 in the topical brimonidine group (p < 0.05). There was a statistically significant decrease in the number of reactive and amoeboid cells (Figures 4A, B).

Figure 4. Compared to sham group (A), the decrease in the number of microglia and change in its morphology in topical brimonidine treatment (B).
Conclusion
Microglia, which are immune system compatible cells of the central nervous system, act as sensors and have neuroprotective properties under physiological conditions. Trauma and degenerative processes cause immune-phenotypic and morphological alterations in microglia, which then respond by proliferating, migrating, and producing inflammatory cytokines. Microglial responses may compromise neuronal survival due to excessive inflammation ıf uncontrolled. A neuroinflammatory response is thought to be one of the etiological causes for a number of neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and glaucoma (14–17). Microglial cells are important players in many neurodegenerative lesions.
Glaucoma, one of the leading causes of blindness in the world, is characterized by the irreversible loss of RGC and loss of visual field. Glial activation has been shown to be important beginning from the first stages of glaucomatous disease (18). It has been shown that active microglia cells play a prominent role in the pathogenesis of glaucoma. Microglia cells can change their morphology, increase in number, migrate, or alter the expression of enzymes, receptors, growth factors, and cytokines. This situation leads to retinal degeneration by causing excessive inflammation (10, 19). Active microglia undergo processes such as withdrawal of extensions, expansion, and growth of the cell body, and expression of myeloid cell markers. In high activation states, microglia cells show amoeboid morphology and do phagocytosis by acting like macrophages (20, 21).
In our study, compared to the control group, a statistically significant increase in the number of Iba-1 positive microglia was observed in sham-treated eyes by creating a transient ischemia model. The concurrent occurrence of a noticeable increase in the amoeboid form supports the triggering of neuroinflammation in the glaucoma model and the generation of amoeboid phagocytic response after ischemia.
Activated microglia have different phenotypic properties, but they also show different functions. M1 microglial cells are amoeboid cells with a high phagocytic capacity. Cytokines such as IFN-γ secreted from damaged cells cause M1-like microglial activation. This phenotype is characterized by the production of TNF-α, IL-1b, IL-6, IL-12, and NO (20, 22, 23). M2-like microglia have a smaller cell body and branched extensions, secrete anti-inflammatory cytokines and neurotrophic factors, and create a protective environment for neurons (23, 24).
Proinflammatory cytokine levels were found to be high in clinical glaucoma studies and experimental glaucoma models. It has been demonstrated in experimental glaucoma studies that active microglia cells can migrate and that they annihilate dead cells (10, 25–27). Amoeboid microglia have been shown to phagocytize damaged axons in human lamina cribrosa (28). In our study, in support of the literature, an increase in amoeboid response was observed in eyes in which transient retinal ischemia was created. However, we do not know the difference between proinflammatory cytokine levels since immunological markers were not examined in our study.
In glaucoma treatment, in addition to the reduction of IOP, agents that have neuroprotective effects, suppress neuroinflammation, and cause immunomodulation are on the agenda. Brimonidine is an antiglaucoma agent that has been shown to have neuroprotective effects in clinical and experimental studies. Many mechanisms of action have been demonstrated when brimonidine is used in the treatment of glaucoma. Although reduction of IOP has neuroprotective effects, this is not the only known mechanism. It has been reported that it has no hypotensive effect when applied systemically in experimental rodent models but has a neuroprotective effect (29). Other possible mechanisms of action examined are inhibition of the apoptotic cascade, reduction of glutamate-induced toxic effects, and increased expression of endogenous brain-derived neurotrophic factor (30–33). There are no studies examining the effects of brimonidine on microglia and neuroinflammation. Ours is the first study that investigates the effects of topical brimonidine treatment on microglia cells. The reduction in the number of astrocytes and Müller cells in immunohistochemical studies and the reduction in glial fibrillar acid protein expression demonstrated that brimonidine reduces gliosis, but their effects on microglia are not clearly known (29, 34, 35).
In studies in experimental glaucoma models similar to ours, a reduction in microglial activation after treatment with minocycline or after a high amount of radiation was observed, and therefore a lower RGC death was observed (26, 36). Besides, a prominent relation has been demonstrated in DBA-2J mice between axon loss in the optic nerve and microgliosis (37).
In our study, observing a significant reduction in the number of reactive and amoeboid-phagocytic cells in rats treated with topical brimonidine demonstrated that brimonidine might have neuroinflammation-reducing effects. Although the increase in the number of microglia in the ramified phenotype suggests that there may be an increase in M2-like active anti-inflammatory microglia, further studies that will be conducted with microglia surface markers are needed to demonstrate this.
The literature demonstrates the importance of immune system cells in glaucomatous neurodegeneration. Glia-induced neuroinflammation has been reported to affect RGC survival. In the new treatment strategies, targeting of the RGC body, dendrite, and axons, glia and microglia, and suppressing neuroinflammation are on the agenda. Further studies are needed to demonstrate specific responses and interactions of immune cells in neurodegeneration.
References
1. Torres-Platas S, Comeau S, Rachalski A, Bo G, Cruceanu C, Turecki G, et al. Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflam. (2014) 11:12.
2. Ramirez A, de Hoz R, Salobrar-Garcia E, Salazar J, Rojas B, Ajoy D, et al. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, parkinson, and glaucoma. Front Aging Neurosci. (2017) 9:214. doi: 10.3389/fnagi.2017.00214
3. Sawada A, Rivera J, Takagi D, Nishida T, Yamamoto T. Progression to legal blindness in patients with normal tension glaucoma: hospital-based study. Invest Opthalmol Vis Sci. (2015) 56:3635.
4. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative normal-tension glaucoma study group. Am J Ophthalmol. (1998) 126:498–505.
5. Tezel G, Chauhan B, LeBlanc R, Wax M. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Opthalmol Vis Sci. (2003) 44:3025.
6. Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Opthalmol Vis Sci. (2004) 45:4049.
7. Ko M, Peng P, Ma M, Ritch R, Chen C. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. (2005) 39:365–73.
8. Zhang S, Wang H, Lu Q, Qing G, Wang N, Wang Y, et al. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Res. (2009) 1303:131–43.
9. Williams P, Marsh-Armstrong N, Howell G. Neuroinflammation in glaucoma: a new opportunity. Exp Eye Res. (2017) 157:20–7.
10. Rojas B, Gallego B, Ramírez A, Salazar J, de Hoz R, Valiente-Soriano F, et al. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J Neuroinflam. (2014) 11:133.
11. Stence N, Waite M, Dailey M. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. (2001) 33:256–66.
12. Soltys Z, Ziaja M, Pawliski R, Setkowicz Z, Janeczko K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res. (2001) 63:90–7.
13. Kreutzberg G. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. (1996) 19:312–8.
15. Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. (2014) 42:1291–301.
16. Glass C, Saijo K, Winner B, Gage F. Mechanisms underlying inflammation in neurodegeneration. Cell. (2010) 140:918–34.
17. MacCormick I, Czanner G, Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomark Med. (2015) 9:691–701.
18. Tezel G, Fourth A. Pfizer ophthalmics research institute conference working group. The role of glia, mitochondria, and the immune system in glaucoma. Invest Opthalmol Vis Sci. (2009) 50:1001.
19. Kreutzberg G. Microglia, the first line of defence in brain pathologies. Arzneimittelforschung. (1995) 45:357–60.
20. Ransohoff R, Cardona A. The myeloid cells of the central nervous system parenchyma. Nature. (2010) 468:253–62.
22. González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. (2014) 274:1–13.
23. Kettenmann H, Hanisch U, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. (2011) 91:461–553.
24. Varnum M, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in alzheimer’s disease brain. Arch Immunol Ther Exp. (2012) 60:251–66.
25. Gallego B, Salazar J, de Hoz R, Ramírez A, Salinas-Navarro M, Ortín-Martínez A, et al. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflam. (2012) 9:586.
26. Bosco A, Crish S, Steele M, Romero C, Inman D, Horner P, et al. Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One. (2012) 7:e43602. doi: 10.1371/journal.pone.0043602
27. Yuan L, Neufeld A. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. (2001) 64:523–32.
28. Neufeld A. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. (1999) 117:1050.
29. WoldeMussie E, Ruiz G, Wijono M. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. (2001) 42:2849–55.
30. Kim H, Chang Y, Kim J, Park C. Alteration of retinal intrinsic survival signal and effect of [alpha]2–adrenergic receptor agonist in the retina of the chronic ocular hypertension rat. Vis Neurosci. (2007) 24:127–39.
31. Hernández M, Urcola J, Vecino E. Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp Eye Res. (2008) 86:798–806.
32. Ma K, Xu L, Zhang H, Zhang S, Pu M, Jonas J, et al. Effect of brimonidine on retinal ganglion cell survival in an optic nerve crush model. Am J Ophthalmol. (2009) 147:326–31.
33. Yiğit U, Erdenöz S, Uslu U, Oba E, Cumbul A, Cağatay H, et al. An immunohistochemical analysis of the neuroprotective effects of memantine, hyperbaric oxygen therapy, and brimonidine after acute ischemia reperfusion injury. Mol Vis. (2011) 17:1024–33.
34. Vidal L, Díaz F, Villena A, Moreno M, Campos J. Reaction of müller cells in an experimental rat model of increased intraocular pressure following timolol, latanoprost and brimonidine. Brain Res Bull. (2010) 82:18–24.
35. Lee D, Kim K, Noh Y, Chai S, Lindsey J, Ellisman M, et al. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. (2012) 7:e47098. doi: 10.1371/journal.pone.0047098
36. Bosco A, Inman D, Steele M, Wu G, Soto I, Marsh-Armstrong N, et al. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Opthalmol Vis Sci. (2008) 49:1437.