Introduction
Vision a key sensory system used in mobility (1, 2). When it is impaired, the ability to obtain information about the environment is reduced, thus affecting the mobility performance (2, 3). Previous studies on low vision have shown that mobility performance is highly dependent on both the level of as well as any transient changes in illumination (2–5). Peripheral vision is an important factor in mobility performance in people with low vision (6). Furthermore, mobility instructors have long recognized that people with peripheral visual field loss show greater mobility difficulty than people with central visual field loss (3, 7, 8). The type of visual field loss and the level of environmental illumination are both well known to influence mobility performance in people with low vision. Mobility is, however, far more complex than these earlier studies suggest. Researchers agree that any definition of orientation and mobility must be considered in the context in which it is being used (6). Orientation may be defined as ‘the process of utilizing all the remaining senses in establishing a person’s position and relationship to all other significant objects in the environment’ (9). However, mobility may be defined as ‘the ability to navigate from one’s present fixed position to one’s desired position in another part of the environment’ (6, 9). Mobility can also be considered to be ‘the ability to perform safe, efficient, and independent travel through an environment and at a speed close to normal walking pace’ (7, 9, 10).
Turano and Wang (11) stated that disease processes may result in the visual system performing anomalous processing of information that may affect visual perception and mobility performance. Visual dysfunction, as seen in RP, will result in a smaller visual field sample and reduce the amount of visual information obtainable in a single fixation (12, 13). This may lead to decreased efficiency in localizing objects or in visual search, resulting in poor orientation and decreased mobility (13). Poor night vision and prolonged dark adaptation in RP can also compound orientation difficulties (14–16). These factors make independent mobility difficult and stressful for people with RP (2, 10, 14, 15) and ultimately decrease their overall level of independence and their ability to travel safely (8, 13). This is a major issue for people with RP. Many studies have been conducted to examine the clinical visual function in people with RP (8, 10, 11, 13, 15). However, relatively few studies have specifically looked at mobility in RP subjects (13, 17, 18). Haymes et al. (17) reported that contrast sensitivity and residual visual field measures account for about 64% of the variance in a multiple regression model of mobility performance in RP. However, the effect of illumination on mobility performance was not investigated in this study. Recently, a few studies have specifically investigated the effect of illumination on mobility performance in RP. Geruschat et al. (8) investigated the relationship between a number of clinical measures and mobility performance under normal and reduced illumination in RP subjects. It was suggested that almost 70% of the variance in the RP subjects’ walking speeds was accounted for by contrast sensitivity and visual field measures. These findings are consistent with previous studies (10, 17). Furthermore, Geruschat et al. (8) suggested that both RP and normally sighted subjects travelled slower under low illumination. This suggests that light levels play an important role in determining the mobility performance of RP subjects. In few studies, the effect of illumination on mobility performance in RP subjects was observed at either “high” or “low” levels. Little quantitative data are available on the effect of illumination on mobility performance in RP subjects. Hence, the investigation of mobility performance as a function of different light levels is of interest.
Therefore, this study aims first to determine the mobility performance of RP subjects compared with age- and gender-matched controls over a range of simulated different illumination levels. The second aim of this experiment is to determine the illumination level at which RP subjects demonstrate increased difficulty in mobility and walking speed indoors. This study may lead to a better understanding of the nature of mobility performance in RP subjects indoors. At the same time, this study could have a considerable impact in determining the minimum level of lighting required by RP subjects indoors to mobilize safely and thus contribute to their quality of life.
Methodology
This was a cross-sectional study. Inclusion criteria include RP subjects diagnosed to have only RP and confirmed by an ophthalmologist, and the age between 18 and 65 years. Previous studies have shown that while there are several genetic (19, 20) and functional subtypes of RP, (21, 22) functional vision loss such as in visual fields, dark adaptation and contrast sensitivity appears to progress similarly regardless of the aetiology. RP subjects with 6/36 acuity or better were included in the study. Any RP subjects with macular changes, central scotoma or constricted visual fields of 5 degrees or less were excluded from the experiment. A total of 21 RP subjects (9 male and 12 female) were recruited from The Retinitis Pigmentosa Society of NSW, The National Foundation of Blind Citizens in NSW and The Low Vision Clinic at the School of Optometry, UNSW. The mean age for the RP subjects was 42.9 ± 10.7 (SD) years with a range of 19–62 years. The visual acuity for the right eye measured with the Snellen chart ranged between 6/5 and 6/36. The mean duration of diagnosis of the RP was 20 ± 12 (SD) years with a range of 2–39 years.
The selection criteria for the control group were aged between 18 and 65 years. All the controls had to be free from any ocular pathology, systemic diseases and congenital colour vision deficiency. To confirm that the control subjects were free from any pathology, ophthalmoscopy, visual acuity and confrontation testing were conducted. The test was conducted only on the right eye. The visual acuity of the control group was 6/5, and no significant lens opacities or macular changes were noted. The control group comprised a total of 21 people (9 male and 12 female) aged 19–63 years. The mean age for the control group was 42.5 ± 11.2 (SD) years. There was no significant difference (t-test, p = 0.91) between the mean ages of the RP and control groups.
In this experiment, two indoor mobility courses were designed to examine the mobility performance of both RP and control subjects. The indoor mobility courses were chosen to measure the mobility performance of these subjects in a controlled environment. The two mobility courses designed were the simple and complex mobility courses. The simple mobility course was conducted under a constant light level, while the complex mobility course was conducted under different simulation light levels. To quantify the mobility performance of the subjects, three mobility indices were measured: (a) preferred walking speed (PWS) – this is a measurement of speed and it assesses the efficiency of independent travel. The score is an indication of the subject’s confidence and indicates the degree of ‘stress’ placed on the subject within the environment (10, 23). (b) Percentage of preferred walking speed (PPWS) – this measurement expresses the subject’s walking speed as a PPWS (10, 23, 24). PPWS is an objective measure of mobility performance that allows more valid inter-subject comparisons, while accounting for the variation in age and physical attributes of subjects (24, 25). PPWS also allows the use of smaller subject samples in experiments, as there is a reduction in inter-subject variation. (18) (c) Mobility incidents – this is a measurement of the number of errors made along a mobility course. It assesses the level of ‘safe’ travel. Any contacts with obstacles are considered ‘unsafe’ mobility (10, 23). The error score is shown as described by Marron and Bailey (7), where Error score = log 10 100/(1 + No. of errors).
Mobility assessments were conducted in two stages. First, a simple mobility course under constant luminance levels (61 cd/m2) was done. Here, the time taken to complete the course was recorded. Subsequently, a complex mobility course was conducted at five different simulated lighting levels, which were 62, 47, 20, 6 and 1 cd/m2. Before performing each test, all subjects were allowed to adapt to the wrap-around spectacles for 10 min each. The time taken to complete each course at each of the light levels was recorded. The time taken by the subjects to complete the complex mobility course at each of the five light levels was measured in seconds, and recorded as PWS (m/s) and expressed as PPWS.
Preferred walking speed at simple course
Informed consent was obtained from all the subjects after an explanation of the nature and possible consequences of the study. The research project was vetted and approved by the University of New South Wales Human Subject Ethics Committee Research and the approval code was CEPIHS No: 97127. In this experiment, the Cronbach’s alpha coefficient of reliability test using SPSS, Student’s t-test, one-way ANOVA, two-way ANOVA with repeated measures on one factor, paired comparison using a t-test with Bonferroni correction (26, 27), Scheffe post hoc comparison (28) and Pearson’s correlation analysis were used to analyse the data.
Results
Preferred walking speed under constant lighting condition
The mean PWS results for the RP and control groups were 1.32 ± 0.14 and 1.37 ± 0.13 m/s, respectively. No significant difference was found between the RP and control groups, both in the time taken (p = 0.25) and PWS (p = 0.21) to complete the simple mobility course. The mean ages of the RP and control groups were 42.9 ± 10.7 and 42.5 ± 11.2 years, respectively. It was found that there was no significant difference (t-test, p = 0.91) between the mean age groups. These findings suggest that RP subjects of similar age groups can travel as fast and as confidently as normally sighted subjects on a straight and unobstructed mobility course.
RP mobility performance under different simulation lighting conditions
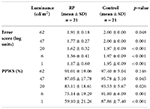
The mean results for the error score and PPWS for RP and control groups are shown in Table 1. Note that a high error score (less mobility incidents) and a high PPWS indicate better performance. Test-retest reliability was conducted for RP and control subjects at different light levels using the Cronbach’s alpha coefficient of reliability. The alpha coefficient is similar to correlation analysis in that the nearer it is to 1, the more reliable the measurement scales. It was found that the alpha coefficient for PPWS was 0.97, and p > 0.0001 for both RP and control groups. The alpha coefficient for error score in the RP group was 0.43, p < 0.01, while for the control group it was 0.90, p < 0.0001. Therefore, it can be concluded that in the PPWS measurement, there was consistency among the individuals in each group. However, for the error score, it is seen that the measurement is significant, although not a very good measure. It is surmised that the small sample size may have influenced this and a larger sample size is indicated to explore the error-score reliability.
Mobility incidents (error score). To determine the mobility performance at different light levels, the error score data were compared between the RP and control groups. The subjects’ mobility incidents were converted into an error score. The mean error scores for RP and control groups are tabulated in Table 1. The data analysis was performed using two-way ANOVA (RP and control groups) with repeated measures on one factor (luminance). This analysis showed an overall significant difference between the RP and control groups (p < 0.0001), indicating that the overall mobility performance of the RP group over all light levels was worse than that of the control group. There was also a significant interaction effect between the two RP and control groups (p < 0.0001) indicating that the two groups did not behave similarly across the luminance levels tested. This finding indicates that the RP subjects tended to encounter increasingly more mobility incidents than control subjects as the light level decreased. A paired comparison using a t-test with Bonferroni correction (α = 0.01) revealed no significant difference between the mean error score for RP and control groups under a normal light level of 62 cd/m2 (Table 1). However, at all other light levels, the mean error scores between the RP and control groups were significantly different. These findings suggest that even in the complex mobility course, RP subjects did not experience any more mobility incidents than controls at normal room light levels. However, once the luminance level decreased, RP subjects experienced a higher number of mobility incidents.
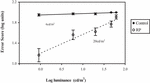
To determine the relationship between error score and light levels, a graph of mean error score against log luminance for RP and control groups was plotted (Figure 1). The Pearson’s correlation analysis showed a significant linear relationship between error score and luminance in RP (r = 0.54, p ≤ 0.001). However, in the control subjects, although there was a slight statistical relationship between error score and luminance (r = 0.21, p = 0.03), the relationship was not considered clinically relevant. To determine the effect of different luminance levels on the error score in each group, repeated measures ANOVA followed by Scheffe’s post hoc comparisons were used. Analysis of the error score for the RP group at different light levels showed that there was a significant difference (ANOVA, p < 0.0001) in the mean error score across light levels, suggesting that the mobility performance of RP subjects was highly dependent on the light level. Scheffe’s post hoc comparisons showed that in the RP group there was no significant difference in the mean error score until the light level decreased below 6 cd/m2. These findings suggest that the RP subjects may bump into more things when the room light level decreases below 6 cd/m2. However, in the control group, there was no significant difference (ANOVA, p = 0.25) in the mean error score with changes in light level.
Walking speed. To determine the mobility performance at different light levels, the PPWS data were compared between the RP and control groups. The subjects’ PWS were converted into PPWS. This allowed more valid inter-subject comparisons. (23–25) The mean PPWS results for the RP and control groups are tabulated in Table 1. The data analysis was performed using two-way ANOVA (RP and control groups) with repeated measures on one factor (luminance). This analysis showed an overall significant difference between the RP and control groups (p < 0.0001), indicating that the overall mobility performance of the RP group over all light levels was worse than that of the control group. There was also a significant interaction effect between the RP and control groups (p < 0.0001), indicating that the two groups did not behave similarly across the luminance levels tested. This finding suggests that the RP subjects travelled more slowly than the control subjects at dimmer light levels. Furthermore, the RP subjects travelled slower than their own PWS when compared to the control subjects at all light levels. Paired comparison using a t-test with Bonferroni correction (α = 0.01) revealed no significant difference between the mean PPWS for RP and control groups under normal light levels of 62 cd/m2 until it was reduced to 20 cd/m2 (Table 1). However, at lower light levels, the means of the two groups were significantly different. These findings suggest that when the room light level ranges between 62 and 20 cd/m2, the mean PWS for both RP and control groups in a complex mobility environment are similar. However, once the light level decreased below 20 cd/m2, the mean PWS for the RP group was slower than the control group.
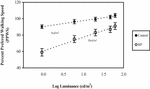
To determine the relationship between the PPWS and light levels, a graph of mean PPWS against log luminance for RP and control groups was plotted (Figure 2). PPWS in the RP group was found to be reduced by almost 35% as the light level decreased from 62 to 1 cd/m2. The reduction in the control group in PPWS was less than 10%. Thus, in darker conditions, both RP and control subjects travelled at a speed slower than their own PWS. However, the RP subjects reduced their walking speed by a much greater proportion than control subjects in similar conditions. The Pearson’s correlation analysis showed a significant linear relationship between the PPWS and luminance in the RP group (r = 0.51, p = < 0.0001). In the control group, there was also some statistical relationship noted between the PPWS and luminance (r = 0.41, p = 0.001).
To determine the effect of different luminance levels on the PPWS in each group, repeated measures ANOVA followed by Scheffe’s post hoc comparisons were used. Analysis of the PPWS for the RP group at different light levels showed that there was a significant difference (ANOVA, p < 0.0001) in the mean PPWS across light levels, suggesting that the mobility performance of the RP subjects was highly dependent on the light level. The Scheffe’s post hoc comparisons showed that in the RP group, there was no significant difference in the mean PPWS until the light level decreased below 20 cd/m2. This finding suggests that the RP subjects may start to have difficulties in mobility performance when the room light decreases below 20 cd/m2. For the control group, there was also a significant difference (ANOVA, p < 0.0001) in the mean PPWS with changes in the light level tested. The Scheffe’s post hoc comparison showed that in the control group, there was no significant difference in the mean PPWS until the light level decreased below 6 cd/m2. This finding suggests that the control subjects may start to have difficulties in mobility performance when the room light level is darker than 6 cd/m2.
Discussion
Mobility performance on a simple mobility course
In the simple mobility course, the PWS was not significantly different between the RP and control groups at the highest light levels (61 ± 6 cd/m2). This result indicates that the RP and control groups have similar mobility performance under normal illumination on a straight and unobstructed route. This is possible because the RP subjects were told that the simple mobility course was obstacle-free and safe for them to travel quickly. This result is consistent with other studies that have reported that on a simple mobility course, RP subjects perform as quickly and safely as control subjects (13, 17).
Mobility performance on a complex mobility course
Mobility incidents
At normal light levels in the complex mobility course, the RP subjects did not experience any more mobility incidents than the controls. However, the RP subjects had almost a 39% decrease in mobility performance when the light level was decreased by almost 2 log units in the same environment. Similar findings have been obtained in previous studies (8, 10). The reduction in the mobility performance of the RP subjects as the light levels decreased in this study clearly demonstrates that the mobility performance of RP subjects is dependent on the lighting conditions. These findings help explain why RP subjects do not have any mobility difficulties, such as bumping into things, under normal light levels but instead have difficulties at lower light levels. This is clearly seen in the relationship between the error score and light levels plotted in Figure 3. From this relationship, it can be seen that the mobility performance of RP subjects can be enumerated quantitatively. It was also found that when the light levels dropped below 20 cd/m2, the RP subjects developed increased mobility difficulties. These findings confirm what was previously a qualitative understanding of the fact that RP subjects do not perform well at low light levels.
A possible explanation for the increased mobility incidents experienced by the RP subjects in low light levels may be due to abnormal rod function (29, 30), which may lead to delayed dark adaptation (31) and reduced mesopic vision (32). Another possible explanation is that it may be due to the reduced amounts of visual information obtainable in a single fixation in these subjects (13). To walk safely, a person must be able to detect potential obstacles, determine their relative location and plan to navigate around them. This task requires data acquisition through successive fixations on the scene. However, with peripheral field loss, there would be a reduced amount of visual information obtainable in a single fixation. This would cause the RP subject to redirect fixation more often and make more eye and head movements. Greater amounts of mental effort would then be required (13). Therefore, in low light levels, the RP subject would not be able to gather all the visual information needed in a similar time frame to the control subjects, causing RP subjects to travel more slowly and experience more mobility incidents than normally sighted people.
Another possible explanation for the increased mobility incidents experienced by the RP subjects is the effect of a reduction in the quality of visual information obtainable in single fixation because of poor contrast sensitivity. It is well documented that contrast sensitivity is affected in RP subjects (33, 34)and that contrast sensitivity is highly correlated with object detection under lower illumination (4). Contrast sensitivity loss may also contribute to difficulties in object detection and recognition, resulting in RP subjects experiencing reduced visual information integration under low light conditions. In the control group, mobility incidents did not show a clinically significant variation with changes in light level. This result is consistent with previous reports (4, 8, 10).
Walking speed
The results of the study of the PWS may be summarised into two parts: (1) the mean PWS of the RP and control groups within simple and complex environments was similar at normal light levels, and (2) in the complex mobility course when the light levels decreased, the mean PWS of the RP group was slower than that of the control group. These findings again support the view that the mobility performance in RP subjects is highly dependent on the light level. These findings are consistent with other studies (8, 10, 17).
The results of the PWS were expressed as PPWS in the complex mobility course; this showed that (1) across all light levels, the mean PPWS in the RP subjects was significantly lower than that in the control subjects, indicating that the RP subjects’ performance was more affected as the light levels decreased. Similar findings have also been noted by previous researchers (10, 24). Haymes et al. (24), in simulated RP subjects, similarly reported that the PPWS was significantly reduced with decreasing retinal illuminance. 2) Under darker conditions, both RP and control subjects travelled slower than their PWS with RP subjects travelling slower. Similar findings were noted by Geruschat et al. (8). A possible explanation for the reduction in the PPWS in RP subjects could be the complexity of the mobility course used. Haymes et al. (25) demonstrated a significant reduction in PPWS when the complexity of a mobility course is increased. Another possible explanation is that the RP subjects were more cautious when travelling along the travel path, especially under different light levels. Besides, the severity of the RP clinical condition may also affect the PPWS. Therefore, all these factors will each contribute individually or collectively to the reduction of the walking speed (PPWS) in RP subjects.
Certain light levels may affect the PPWS in RP subjects more than in the control subjects. This can be seen clearly in the relationship between the PPWS and the light levels plotted in Figure 4. From the relationship, it is seen that the mobility performance (PPWS) of the RP subjects can be enumerated quantitatively. It was observed that the RP subjects started to experience difficulty when the light levels decreased by about 0.5 log luminance, while the control subjects started to experience difficulty when the light levels decreased by about 1.0 log luminance from normal room lighting. This differential light threshold is an important finding of this study. These findings confirm what was previously only a qualitative understanding of the fact that RP subjects do not perform well at low light levels. For example, when the RP subjects finish hanging clothes outdoors on a bright sunny day and then enters indoors, it would take a while for the RP subjects to be able to see clearly indoors. It is probably a good idea then to switch on the lights first before entering the kitchen so that there will be a lesser change in the ambient light levels. Due to the limited number of RP subjects, further investigation with a larger population of RP at different stages of the disease (early, moderate and advanced) according to visual field size should be conducted to confirm the current findings and minimise the effect of diverse populations.
Conclusion
The purpose of this study was to determine the relationship between light level and mobility performance in RP subjects. It can be concluded that at high constant light levels and on a simple mobility route, the mobility performance of the RP and control groups was similar. This finding illustrates that RP subjects do not have any difficulties in mobility when they are in normal lighting conditions. However, at different light levels and in the complex mobility route, a 2.0 log unit decrease in luminance had a strong, adverse effect on the mobility performance of the RP subjects. This is shown in the relationship between the mobility indices (PPWS and error score) and luminance. Clinically, measuring mobility incidents remains important. It is socially awkward making unwanted contact while walking. Furthermore, it is possibly a threat to one’s own safety. Therefore, mobility incidents should be considered carefully when determining the mobility performance of the RP subjects. In this study, the mobility performance of the RP subjects could be enumerated quantitatively. It was found that the relationship between PPWS and luminance in the RP subjects was linear. The PPWS of the RP subjects decreased significantly when the path luminance dropped below 20 cd/m2. The error score was also found to be linearly related to log luminance. The complexity of the mobility route will also affect the mobility performance of the RP subjects, and this can be seen with the increased number of mobility incidents, especially at low light levels.
Clinically, these findings will be able to help clinician in explaining to the RP subjects or their guardians why certain light conditions will have an adverse effect on the RP subjects while with other light conditions, the RP subjects are not affected, especially indoors. For example, when a patient with RP walks into a theatre, the RP subject will need some time to adjust to the dim light levels in the theatre before he or she can see clearly enough to locate their seats. In outdoors, the RP subjects may also experience difficulties, such as when a patient with mild RP drives into and then out of a tunnel. The RP subject must be extra careful when driving because it will take a while for them to adapt to different light levels. Furthermore, these findings also help the clinician to determine the minimal lighting levels that should be used in the modification of the environment for the RP subject so that mobility problems and other issues may be reduced. For the control subjects, a 2.0 log unit decrease in luminance had little effect on the mobility performance. The PPWS was essentially independent of luminance, although a slight decrease in PPWS was noted when the luminance dropped below 6 cd/m2. The error score was also essentially independent of log luminance, and the complexity of the mobility route had no effect on the mobility performance.
Acknowledgments
I would like to thank all the RP subjects who participated in this study.
References
1. Pelli D. The visual requirements of mobility. In: GC Woo editor. Low Vision. New York, NY: Springer (1987). p. 134–46.
2. Kuyk T, Elliott J, Biehl J, Fuhr P. Environmental variables and mobility performance in adults with low vision. J Am Optom Assoc. (1996) 67:403–9.
3. Kuyk T, Elliott J, Fuhr P. Visual correlates of mobility in real world settings in older adults with low vision. Optom Vis Sci. (1998) 75:538–47.
4. Cornelissen F, Bootsma A, Kooijman A. Object perception by visually impaired people at different light levels. Vis Res. (1995) 35:161–8.
5. Cornelissen F, Kooijman A, van Schoot E. Optimizing illumination for visually impaired persons. Low Vis Res New Dev Rehabil. (1994) 11:68.
6. Lovie-Kitchin J, Mainstone J, Robinson J, Brown B. What areas of of the visual field are important for mobility in low vision patients. Clin Vis Sci. (1990) 5:249–63.
7. Marron J, Bailey I. Visual factors and orientation-mobility performance. Am J Optom Physiol Optics. (1982) 59:413–26.
8. Geruschat D, Turano K, Stahl J. Traditional measures of mobility performance and retinitis pigmentosa. Optom Vis Sci. (1998) 75:525–37.
9. Apple L. Orientation and Mobility of Patients with Low Vision. Clinical Low Vision. Boston, MA: Little, Brown and Company (1976). p. 137–40.
10. Black A, Lovie-Kitchin J, Woods R, Arnold N, Byrnes J, Murrish J. Mobility performance with retinitis pigmentosa. Clin Exp Optom. (1997) 80:1–2.
11. Turano K, Wang X. Motion thresholds in retinitis pigmentosa. Investig Ophthalmol Vis Sci. (1992) 33:2411–22.
13. Turano K, Geruschat D, Stahl J. Mental effort required for walking: effects of retinitis pigmentosa. Optom Vis Sci. (1998) 75:879–86.
16. Omar R, Herse P. Quantification of dark adaptation dynamics in retinitis pigmentosa using non-linear regression analysis. Clin Exp Optom. (2004) 87:386–9.
17. Haymes S, Guest D, Heyes A, Johnston A. Mobility of people with retinitis pigmentosa as a function of vision and psychological variables. Optom Vis Sci. (1996) 73:621–37.
18. Turano K, Geruschat D, Stahl J, Massof R. Perceived visual ability for independent mobility in persons with retinitis pigmentosa. Investig Ophthalmol Vis Sci. (1999) 40:865–77.
19. Foxman S, Heckenlively J, Bateman J, Wirtschafter J. Classification of congenital and early onset retinitis pigmentosa. Arch Ophthalmol. (1985) 103:1502–6.
20. Farrar G, Jordan S, Kumar-Singh R, Inglehearn C, Gal A, Greggory C, et al. Extensive genetic heterogeneity in autosomal dominant retinitis pigmentosa. In: JG Hollyfield, RE Anderson, MM LaVail editors. Retinal Degeneration. Boston, MA: Springer (1993). p. 63–77.
21. Fishman G, Alexander K, Anderson R. Autosomal dominant retinitis pigmentosa: a method of classification. Arch Ophthalmol. (1985) 103:366–74.
22. Jacobson S, Voigt W, Parel J, Apathy P, Nghiem-Phu L, Myers S, et al. Automated light-and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. (1986) 93:1604–11.
23. Clark-Carter D, Heyes A, Howarth C. The efficiency and walking speed of visually impaired people. Ergonomics. (1986) 29:779–89.
24. Haymes S, Guest D, Heyes A, Johnston A. Comparison of functional mobility performance with clinical vision measures in simulated retinitis pigmentosa. Optom Vis Sci. (1994) 71:442–53.
25. Haymes S, Guest D, Heyes A, Johnston A. The relationship of vision and psychological variables to the orientation and mobility of visually impaired persons. J Vis Impair Blind. (1996) 90:314–24.
26. Stevens J. Intermediate Statistics: A Modern Approach. Hillsdale, NJ: L. Erlbaum Associates (1990).
29. Kemp C, Jacobson S, Faulkner D. Two types of visual dysfunction in autosomal dominant retinitis pigmentosa. Investig Ophthalmol Vis Sci. (1988) 29:1235–41.
30. Kemp C, Jacobson S, Roman A, Sung C, Nathans J. Abnormal rod dark adaptation in autosomal dominant retinitis pigmentosa with proline-23-histidine rhodopsin mutation. Am J Ophthalmol. (1992) 113:165–74.
31. Moore A, Fitzke F, Chen J, Kemp C, Bird A. Prolonged rod dark adaptation in autosomal dominant sector Retinitis pigmentosa. Invest Ophthalmol Vis Sci. (1989).
32. Alexander K, Derlacki D, Fishman G, Peachey N. Acuity-luminance and foveal increment threshold functions in retinitis pigmentosa. Investig Ophthalmol Vis Sci. (1991) 32:1446–54.
33. Spellman D, Alexander K, Fishman G, Derlacki D. Letter contrast sensitivity in retinitis pigmentosa patients assessed by Regan charts. Retina. (1989) 9:287–91.