Introduction
Though cancer is rare, approximately 400,000 children (0–19 years) develop cancer each year.(1, 2) In the United States, the incidence rate of childhood cancer in 1975 was about 0.8% per year, which showed an increasing trend from 1997 to 2018 and 1% (3). After an accident, childhood cancer is the 2nd most common cause of child death in children under 15 years of age (4). According to the American Cancer Society, in 2022, there will be about 3,360 new cases of eye cancer in the United States (5). In the United Kingdom, eye cancer is not among the 20 most common cancers, accounting for less than 1% of all new cancer cases (2016–2018) (6). In India, the incidence of eye cancer is estimated to be < 0.5% (7). Pediatric ocular tumor is less common in comparison to the adult tumor and also varies in type. One study from the United Kingdom showed the annual incidence of eye cancer under 15 years was 3.5/1,000,000, but it was 11.8/1,000,000 in the case of under 5 years (8). Benign tumors are more common in children than malignant ones. Metastatic tumors also affect them, and the common site of deposition is orbit. Dermoid cysts, retinoblastoma, and leukemia are the most commonly benign, malignant, and metastatic ocular tumors in children, respectively.
Common childhood tumors with their epidemiology, presentation and recent management are reviewed here.
Classification
There are differences in the site of origin and the time of presentation of ocular tumors between the pediatric group and adults. Some tumors are congenital and some are acquired. Although congenital tumors usually appear at birth or shortly after birth, they may present lately when a complication arises. The pediatric tumor can be classified as a tumor or tumor-like lesion of orbit, intraocular tumor, and surface tumor (Tables 1–3).
Intraocular tumor
Retinoblastoma
Retinoblastoma (RB) is the most common primary intraocular malignancy in children worldwide. It originated from the primitive retinoblastoma, which arise from the inner neuroepithelial layer of the embryonic optic cup. The incidence is 1: 16,000 to 1: 18,000 live births, (11) and about 9,000 new cases are detected every year. Although this tumor is found in all continents, 43% of global burden lives are in 6 Asian countries (India, China, Indonesia, Pakistan, Bangladesh, and the Philippines) (12).
The tumor may arise at any age, but the median age at diagnosis is 1.5 years (18 months). According to the Retinoblastoma Collaborative Study, about 90% of cases are detected before the age of 6 years. This tumor is occasionally detected as a congenital or even as an intrauterine disorder (13). It may be heritable or non-heritable and may be present as bilateral (40%) or unilateral (60%), with multifocal or unifocal locality according to its heritance and penetration. Though improved treatment facilities have increased the survival rate by more than 95% in developed countries, (14) retinoblastoma is still deadly cancer worldwide, with an estimated death rate of more than 40% and most of which are reported from Africa and Asia.
Retinoblastoma is an example where the genetic nature of cancer was revealed (15). It arises due to the inactivation of both the alleles of the Rb1 gene, which are located on the 13q14 chromosome. A recent study shows that retinoblastoma may differ in mutagenic pathways, for example, some retinoblastoma tumors can be caused by Rb1 mutation (15) and others by amplification of MYCN gene (16). The most common presenting sign of retinoblastoma is leukocoria (Figure 1). The others are strabismus, hyphemia, hypopyon, secondary glaucoma, orbital cellulitis, proptosis, phthisis bulbi, etc. Usually, children from underdeveloped countries present with more advanced stages than those from the developed world. The heritable retinoblastoma usually presents as a bilateral or unilateral tumor at an early age, but multifocal tumors and non-heritable tumors present as unilateral and unifocal tumors at a later age. There are some other causes of leukocoria that may confuse this devastating cancer. The most common differentials are persistent hyperplastic primary vitreous (PHPV), coat’s disease, ocular toxocariasis, and retinopathy of prematurity (ROP) (17). A complete ophthalmic examination with full mydriasis under general anesthesia is needed to properly diagnose the disease. An imaging study is needed for the appropriate grouping and staging of the tumor and the planning of management.
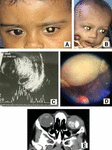
Figure 1. Retinoblastoma. (A) Unilateral RB, (B) Bilateral RB, (C) B-Scan showing intraocular mass lesion with calcification, (D) Group C tumor, (E) CT scan showing bilateral intraocular RB with calcification.
The intraocular tumor is grouped after examining the child under general anesthesia with an indirect ophthalmoscope. There are several classifications for grouping and staging, but the International Retinoblastoma Classification (Group A to E) is most commonly followed. To identify the extraocular extension, intracranial metastasis, and pineoblastoma, different imaging techniques such as ultrasonography (US) (B-Scan), CT scan, and MRI are used. The US (B-Scan) demonstrates RB as an intraocular mass with characteristic calcifications as indicated by high internal reflectivity (Figure 1). Calcification is also found in CT scans, and if it is present in children below the age of 3 years, retinoblastoma should be suspected (Figure 1). Though CT scan causes low-dose radiation, which is a risk for a second malignant neoplasm in heritable retinoblastoma, spiral CT can be considered over conventional CT due to its less radiation exposure (18). MR imaging with fat suppression and contrast demonstrates the optic nerve involvement, the spread of tumors outside the globe, intracranial metastasis, and pineoblastoma.
The management plan for retinoblastoma depends on the presentation, such as whether it is bilateral or unilateral, intraocular or extraocular, and its extent of metastasis. In recent years, retinoblastoma has been treated with a multidisciplinary team approach comprising ocular oncologists, pediatric oncologists, radiation oncologists, pediatricians, ocular pathologists, ocularists, and genetists (19).
There are different treatment modalities to treat retinoblastoma as follows: (19).
(A) Local therapy
(i) Laser photocoagulation (Argon/YAG laser)
(ii) Transpupillary thermotherapy (diode laser)
(iii) Cryotherapy
(B) Chemotherapy
(i) Local chemotherapy—periocular, intravitreal, and intracameral
(ii) Systemic chemotherapy
(iii) Intra-arterial chemotherapy
(iv) Intrathecal chemotherapy
(C) Radiation therapy
(i) Plaque radiation therapy (Brachytherapy)
(ii) External beam radiation therapy
(iii) Proton beam therapy
(D) Surgery
(i) Enucleation (intraocular)
(ii) Exenteration (extraocular)
Currently, one of the main management protocols for retinoblastoma is intravenous chemotherapy in conjunction with local therapy, and most oncology centers practice the three-regimen therapy using vincristine sulfate, etoposide phosphate, and carboplatin for 6 cycles every 3–4 weeks (20) for intraocular tumors and 12 cycles for extraocular tumors. Intravenous chemotherapy is indicated in bilateral cases; unilateral cases grouped as B, C, and D; histopathological high-risk cases; and extraocular cases. These chemotherapeutic agents have some toxicities, such as a decrease in all blood cell count (cytopenia) in 89% of cases, neurotoxicity due to vincristine in 40% of cases, fever due to decreased neutrophil count in 28% of cases, increased rate of infection in 9% of cases, neurotoxicity due to vincristine in 40% of cases, and others like gastrointestinal disturbance and dehydration (21).
Transpupillary thermotherapy (TTT) is currently the most commonly used adjuvant therapy. Treatment can be undertaken alone as a primary treatment (for a very small tumor of <3 mm in diameter) or in combination with chemotherapy. Laser photocoagulation is infrequently used today as it coagulates the vascular supply, decreasing the delivery of chemotherapeutic agents to the eye. Cryotherapy is also frequently used for peripheral tumors under 3 mm in greatest dimension and subretinal seeds. A triple freeze-thaw technique is used and the tumor control rate is up to 90% (19).
Local chemotherapies are used to control the recurrent and residual vitreous seeds along with systemic chemotherapy to increase the vitreous concentration without increasing the dose of systemic chemotherapy, thus preventing some serious adverse effects. Intra-arterial chemotherapy is used as a primary treatment or in intravenous chemo-resistant cases. Carboplatin, topotecan, and melphalan are used as chemotherapeutic agents and need intervention by a radiologist for this procedure. Along with the toxicities of chemotherapeutic agents, its procedure for drug delivery can cause devastating ocular complications like central artery occlusion.
Plaque radiotherapy with I-125 and Ru-106 is localized radiotherapy and is commonly used secondarily to treat tumors that have failed other focal therapies. It may also be used as a primary treatment modality. The most commonly known external beam radiation (EBRT) therapy has less use now as there are reports of local adverse effects and chances of second malignancy in the irradiated area even after long days, especially in the case of hereditary RB. This type of radiotherapy has been advocated in certain situations following enucleation for RB, most notably for orbital recurrence, involvement of surgical margin of the optic nerve, and diagnosed as orbital RB (22). A total of 45 Gray doses are given to the targeted area in a divided dose for 5 weeks. A new modality of radiation therapy that is proton beam radiotherapy (PBRT) shows better target volume coverage while sparing non-target structures but is highly costly.
In recent years, the frequency of primary enucleation has decreased tremendously, but in underdeveloped and developing countries, enucleation is still the prime treatment modality. Enucleation is usually done in primary uniocular Group-E retinoblastoma and non-responding or poorly responding tumors of bilateral disease. During enucleation, minimal manipulation and ‘no-touch’ surgical techniques are practiced with the resection of a long optic nerve stump, preferably more than 10 mm, which is recommended. After enucleation, the eye should be examined macroscopically to see if there is any optic nerve abnormality and extraocular extension. All specimens should be sent for histopathological examination and also need collaboration with a histopathologist. Per operative use of integrated or non-integrated orbital implant and postoperative use of prosthesis usually 6 weeks after enucleation will increase the cosmetic appearance of children.
Along with the treatment, routine follow-up, screening of siblings, and genetic counseling are mandatory. Following tumor regression, subsequent examination of children should be 3 monthly in the first year, 4 monthly in the second year, 6 monthly up to the age of 6 years, and after that, yearly (19).
Screening for any mutation of the RB1 gene from both tumor and peripheral blood can identify the germline mutation, which may help in proper management planning for both the affected child and sibling (23). Genetic counseling supports families to understand medical diagnosis, disease prognosis, and risk of transmission of disease to offspring. The RB survivors need both visual and psychosocial rehabilitation by spectacles, low vision aids, cataract surgery, and custom-made prostheses.
Medulloepithelioma
Intraocular medulloepithelioma is a congenital tumor that arises from the primitive medullary epithelium. The non-pigmented portion of the ciliary body is the main area of origin of the tumor but rarely other areas of the eye can be affected such as the iris, retinal stalk, and optic nerve (24). It usually presents unilaterally and in 75–90% of cases by the first decade of life, though adults can also be affected (24). This tumor is slow growing and histologically may be benign, malignant, and teratoid or non-teratoid (25).
Patients present with leukocoria and usually with secondary effects of large tumors such as loss of vision, pain, cataract (sectoral or total), retrolental membrane, and neovascular glaucoma. Slit lamp examination reveals medulloepithelioma as pinkish-gray-colored intraocular mass with a smooth surface but irregular in shape. It may be associated with conjunctival injection, prominent episcleral vessels, corneal stromal edema, deposition on the iris, ectropion uvea, and lens subluxation. In 50% of cases, the tumor has an intratumoral cyst, which may float in the anterior chamber and is highly suggestive of a medulloepithelioma. Rarely, it may be pigmented. Most medulloepithelioma are cytologically malignant, but rarely distance metastasis can happen and only lymph node metastases can occur in the region of the neck with a predilection to the parotid gland (24, 25).
A literature search showed some association of ciliary medulloepithelioma with a rare variety of malignant lung neoplasms known as pleuropulmonary blastoma (PPB), which manifests as an innocent-looking lung cyst. This neoplasm also arises from embryonal cells (26). Genetic mutation is found in the DICER1 gene in both diseases (26).
Ultrasound B-scan, ultrasound biomicroscopy (UBM), anterior segment optical coherence tomography (AS-OCT), CT, and MRI are used to diagnose this tumor. B-scan demonstrates ciliary body mass with high echogenicity but heterogeneity. It also demonstrates intratumoral irregular cystic spaces. AS-OCT and UBM can detect the radial expansion and height of lesions in the ciliary body with a demonstration of cysts. The standard diagnostic tool is MRI which demonstrates both cystic and solid components within the tumor as a heterogeneous mass which is located behind the lens (27).
Treatments for ciliary body medulloepithelioma are cryotherapy, plaque radiotherapy, external beam radiotherapy, local resection, and enucleation. Enucleation is a standard treatment protocol in advanced cases with large tumors and neovascular glaucoma. In cases with orbital extension, exenteration and extended enucleation are performed. All surgeries should be performed with minimal handling. Cryotherapy, radiotherapy, and local resection are the treatment options for small tumors which do not exceed 3–4 clock hours. Recurrent tumors can also be treated with cryotherapy. Local resection tends to recur. I-125 and Ru-106 are the plaque brachytherapy agents used to treat small- to medium-sized tumors. In metastatic cases or tumor that arises from the optic nerve, a combination of chemotherapy, brachytherapy, enucleation, or exenteration may need. Chemotherapy includes a combination of ifosfamide, carboplatin, and etoposide (24, 25).
There is often a delay in the diagnosis of this tumor due to its secondary effects, such as glaucoma, sectoral cataract, and uveitis. Due to misdiagnosis, some patients may be treated with surgery, which increases the possibility of extraocular spillage of the tumor and affects the prognosis.
Retinal astrocytic hamartoma
A benign glial tumor arises from retinal astrocytes as multiple or solitary lesions. The appearance is creamy-white, elevated, well-circumscribed, either small, smooth, flat, non-calcified, or mulberry in appearance, which are yellow-white calcified lesions. Tuberous sclerosis (TS) is associated with a retinal astrocytoma in 50% of cases, and if astrocytoma is diagnosed, tuberous sclerosis should be searched. The incidence of TS is 1 in 10,000 (28). This may be rarely associated with neurofibromatosis. Diagnosis is done clinically and to evaluate macular edema and subretinal fluid, the use of optical coherence tomography is beneficial.
Observation is enough in most cases as these lesions create no clinical symptoms. These tumors may resolve spontaneously but become symptomatic when enlarged in size, the occurrence of macular edema, lipid exudation, or serous retinal detachment, and are associated with raised intraocular pressure. If macular edema or exudative detachment does not resolve spontaneously within 6 weeks, treatment is indicated (25).
Retinal hemangioblastoma
It is a vascular hamartoma associated with Von Hippel-Lindau (VHL) syndrome. It was previously known as retinal capillary hemangioma. The age of presentation is in the first two decades of life and involves bilaterally multiple lesions. Patients with retinal hemangioblastoma should be evaluated for brain and spinal cord hemangioblastoma and renal, pancreatic, and ear lesions. This is genetically an autosomal-dominant disease due to a mutation of the VHL gene located on chromosome 3p, and family members should be screened. For detection and confirmation of retinal hemangioblastoma, fluorescent angiography (FA) is the best test as it shows rapid filling of the feeding artery, then the tumor, followed by the rapid exit through the draining vein (29). Management should include both systemic and ocular evaluation. Complete dilated funduscopic examination and FA should be done from 5 years of age and younger children to detect pinpoint tumors in patients with positive family history. Treatment of the tumor depends on the clinical situation (29). If the tumor is associated with VHL syndrome, it tends to be more aggressive and nearly all tumors must be considered for treatment. In small (<3 mm) lesion laser photocoagulation or photodynamic therapy (PDT), in medium (3–6 mm) lesion PDT or cryotherapy, and in large (>6 mm) size PDT, plaque radiotherapy, or internal resection by pars plana vitrectomy route can be employed. In asymptomatic cases where the tumor is small, without subretinal fluid, and not associated with VHL syndrome, it can be observed. Treatment should be commenced if leakage ensues. Patients need multidisciplinary management with an ophthalmologist, neurosurgeon, internal medicine specialist, and ENT specialist.
Rhabdomyosarcoma
Rhabdomyosarcoma (RMS) is a soft tissue sarcoma and a highly malignant tumor originating from pluripotent mesenchyme (30, 31). This disease usually presents first to an ophthalmologist. It most commonly occurs in the head and neck, with 10% of cases occurring in orbit. This tumor typically appears within 10 years of age, but in the literature review, it was reported to be found at birth as a congenital tumor and even at 80 years of age (32, 33). The mean age of onset is 7–8 years and 90% of primary orbital RMS are presented before 16 years. The male-to-female ratio is 5:34 (34). Primary orbital rhabdomyosarcoma commonly affects the orbital cavity and rapid onset (80–100% of cases) of proptosis (Figure 2A-1) is the typical presentation. Proptosis is non-axial with downward and outward (30) displacement of the globe found in 80% of cases (30–32). Other sites of RMS are eyelids and conjunctiva, and on rare occasions, the uveal tract may also be involved. The lid involvement is presented as eyelid edema with ptosis and sometimes atypical presentations such as eyelid nodules (35). Orbital bones can be invaded by orbital rhabdomyosarcoma. Intracranial spread may happen in adults and, in some cases, radiotherapy along with chemotherapy is not able to prevent it (31, 36). In orbital RMS, the metastatic spread is unusual. The first 3 years after treatment is a risky period for recurrence or metastasis. Metastasis commonly occurs in the lungs, (37) and other sites are bone and bone marrow through hematogenous routes (32).

Figure 2. Orbital tumors. (A-1,A-2) Rhabdomyosarcoma presented as proptosis with CT scan image. (B-1,B-2) Leukemic infiltration as proptosis and bilateral lid swell. (C-1,C-2) Neuroblastoma presented as unilateral proptosis and adrenal mass in CT scan.
A thorough history of any child under 2 years of age presenting with a mass in orbit is essential (31). Imaging is also necessary for diagnosis. In suspicious cases, a tissue biopsy must be done.
Immunohistochemical studies establish the primary method of diagnosis. To evaluate the extent of the tumor, staging of the tumor, and proper follow-up, MRI and CT scans are necessary (38). Bone involvement is better seen in CT scans, and soft tissue involvement and intracranial spread are better seen in MRI (39). The CT scan of an orbital RMS at an early stage appears as a homogeneous, isodense, soft tissue mass, not deformed by bone, but in more advanced cases is present with bone destruction and calcification (38–40). The Intergroup Rhabdomyosarcoma Study(IRMS) has currently staged RMS as Group 1 to Group 5. A multidisciplinary approach is essential for treating orbital RMS. Several studies have introduced different techniques, and they suggest combining surgery to remove all tumors or as much as possible with the addition of chemotherapy and radiotherapy according to the staging of the disease (37). Previously, orbital RMS was primarily treated by exenteration. The overall 5-year survival rate in children is about 70%, while it is lower in adults.
Orbital dermoid
Dermoid is a benign congenital choristoma formed by the sequestration of ectoderm during embryological development (41). The condition is the most common benign childhood tumor and accounts for 35–47% of all childhood orbital lesions and 89% of all cystic orbital lesions (42). According to Sherman et al., superficial dermoid typically presents in infancy, but deep orbital dermoid usually presents at the age of about 18 (43).
A dermoid cyst in orbit is usually associated with the zygomaticofrontal and fronto-ethmoidal sutures (44). Based on their relationship to the interzygomatic line on the CT scan, superficial and deep cysts are all classified according to their relationship with suture lines (45–49).
In addition to cosmetic deformity, dermoid cysts cause mechanical ptosis and proptosis (Figure 3). A ruptured cyst can cause pain, swelling, and redness due to inflammation. Histopathological examination shows a keratin-filled cyst bordered by stratified squamous epithelium. It may also contain dermal appendages like hair follicles, sweat, and sebaceous glands (41, 50).
The definitive treatment is to complete surgical excision without disrupting the cyst wall to avoid recurrence (47). Angular dermoid is surgically approached through the lid crease to minimize the ugly scar and for a better cosmetic outcome.
Plexiform neurofibroma
Plexiform neurofibroma (PNF) is a neuroectodermal hamartoma, and it represents 1–2% of all orbital tumors. PNF is diagnostic of neurofibrosis (NF)-1, genetically autosomal dominant, and usually presents within the first 10 years of age (51).
Any peripheral nerve is affected by the lesion, but sensory nerves of the orbit and eyelid are usually involved. Orbital lesions may cause proptosis, bony expansion, and dysplasia of the sphenoid bone, which may cause temporal lobe herniation and pulsatile proptosis. Eyelid PNFs are configured like ‘S’ due to the thickening of nerve, fat deposition, and horizontal redundancy. The lesion feels soft, and it is described as “a bag of worms” (25). There is a gradual increase in lesions causing ptosis of variable degrees and even facial disfigurement. Patients need regular ophthalmic examination as there is a chance of amblyopia, anisometropia, strabismus, ipsilateral glaucoma, and optic nerve glioma (Figure 4). PNF has a 7–10% risk of malignant transformation to sarcoma (52).

Figure 4. NF-1: (A) Associated with right eye proptosis with a squint, (B) disc swelling, and (C) CT showing fusiform thickening of the optic nerve.
Diagnosis is mainly done based on the history and examination of the patient. CT or MRI can help in determining the tumor extension, bony abnormality, and prolapse of meninges or brain tissue through the bony gap, which also helps in planning for surgery. Treatment is to relieve the specific symptoms by surgery, but only debulking can be done, not complete removal due to its infiltrative nature. As PNF is progressive in nature, multiple surgical sessions may be needed.
Neuroblastoma metastases
Neuroblastoma arises from neural crest cells of the adrenal gland (most frequently) (Figure 2C-2), neck, chest, abdomen, or spine and is the most common primary childhood tumor that metastasizes to orbit (53). Children below 4 years are usually affected, and the incidence is approximately 1–3 in 100,000 cases. Neuroblastoma constitutes 6–10% of all pediatric tumors and about 15% of tumor-related deaths in children (54). In 1–2% of cases, neuroblastoma may be inhibited as autosomal dominant (55). Orbital metastasis is most commonly present as periorbital and eyelid ecchymosis, which is known as raccoon eyes. The second common presentation is unilateral or bilateral proptosis (Figure 2C-1). Primary orbital presentation is found in 8% of cases (53). Periocular edema, subconjunctival hemorrhage, vision loss, or decreased ocular motility are some of the less common presentations. These symptoms may be associated with pancytopenia, anemia, fever, abdominal pain, and hepatosplenomegaly. Patients may present with Horner’s syndrome if the tumor arises from the sympathetic chain that is in thoracic neuroblastoma. Diagnosis is made based on history, clinical examination, and the exclusion of recent trauma. Imaging (CT or MRI) of the orbit and brain will show the preference of involvement for the posterolateral orbital wall, and metastasis appears as a well-circumscribed or ill-defined lesion. PET/CT is now shown to be successfully staging and monitoring the disease by improving the detection of smaller lesions and providing anatomic details for surgical planning (56). Urinary and serum catecholamines, including homovanillic acid (HVA) and vanillylmandelic acid (VMA), were previously thought of as more specific and sensitive, but recent studies have shown they are not always present and are not so much sensitive (57).
Treatment started with stratification, which depends on the age of presentation, staging of the tumor, and tumor-specific biological markers. The tumor-specific markers are based on histopathological analyses, chromosomal abnormalities, and quantification of the expression of the MYCN oncogene.
Treatment consists of a combination of chemotherapy, radiation therapy, surgery, myeloablative therapy with stem cell transplant, and immunotherapy such as anti-GD2 monoclonal antibody therapy (58). These multimodality treatments have increased the survival rate. Children below 1 year of age showed a better prognosis, that is, a 5 year survival rate is 80% in comparison to above 1-year-old children where a 5 year survival rate is 45%.
Leukemia
Leukemia can affect any part of the eye, such as the ocular adnexa, conjunctiva, sclera, cornea, anterior chamber, iris, lens, vitreous, retina, choroid, and optic nerve. Ophthalmic involvement can be (1) primary or direct leukemic infiltration in orbit, anterior segment, and central nervous system (Figures 2B-1,2) and (2) secondary or indirect involvement of the retina and vitreous due to anemia, thrombocytopenia, hyperviscosity, and immunosuppression (59).
Acute lymphoblastic leukemia (ALL) is the commonest form of leukemia in children, but in cases of ocular involvement (orbital or intraocular), acute myeloid leukemia (AML) is found more (66.6%) than in those with ALL (15.1%) (60). Granulocytic sarcoma is a rare solid tumor found within orbit which is made up of primitive granulocyte precursors in AML. This isolated orbital mass may appear before a diagnosis of AML by hematological examination. It may also appear in orbit in established AML or may be a sign of relapse in previously treated patients. In 90% of cases, the mean presenting age is 8–9 years with unilateral disease (25). Leukemic retinopathy that is flame-shaped hemorrhages, sometimes with white centers within the nerve fiber layer, is the commonest presentation. Other intraocular presentations are perivascular infiltrations, microinfarctions, serous retinal detachments, hyphemas and pseudohypopyon, and iris masses. Papilledema is the optic nerve infiltration secondary to CNS leukemia. Orbit is less commonly affected, and rapidly enlarging orbital mass is the common presentation, which can cause pain, eyelid swelling, ecchymosis, diplopia, and proptosis (25). Literature review showed children with acute leukemia, if present with ocular involvement, is associated with poor prognosis. So ocular examination should be done on these patients to assume the prognosis (59).
Diagnosis is made by history of the systemic association, clinical examination, and complete blood test with a peripheral blood film, which gives important clues in previously undiagnosed cases. Diagnosis is confirmed by bone marrow study. In granulocytic sarcomas, blood examination usually shows no clues, and in such cases, a biopsy should be done. CT scan or MRI can be done to see the extent or to exclude other differentials. Treatment needs a combination of a pediatric oncologist, pediatrician, radiation oncologist, and ophthalmologist.
On basis of specific genetic features of the malignancy, the treatment of leukemia is customized and the options are chemotherapy and bone marrow transplantation. Optic nerve infiltration is a medical emergency, and vision may be permanently lost if treatment is not done. Immediate low-dose radiotherapy is the treatment choice, which is often added with intrathecal chemotherapy.
Optic pathway glioma
Optic pathway glioma (OPG) is categorized as juvenile pilocytic astrocytomas and accounts for 4–6% of all brain tumors in children (25). It is a low-grade neoplasm arising from the pre-cortical optic pathways and can involve the optic nerve, optic chiasm, optic tracts, optic radiations, or the hypothalamus. Children below the age of 10 years are mostly affected and account for 71%. This tumor either arises sporadically or is associated with neurofibromatosis type 1 (NF1) which is autosomal dominant (61). About 15–20% of patients with neurofibromatosis type 1 usually develop optic pathway glioma, but symptoms develop only in 30–50% of these affected patients.
As OPG is slow growing, the clinical manifestation may be delayed and the usual presenting symptom is a decrease in vision. Other findings are edema of the optic disc, disc pallor, optic atrophy, defective color vision, defect in the visual field, proptosis, strabismus, and defective ocular motility (25) (Figures 4A, B). In 25% of cases, the OPG is confined to the optic nerve, but the rest of the 75% show chiasm involvement, and 40% of chiasm tumors may extend up to the hypothalamus or third ventricle (62).
CT scans and MRIs show characteristic features, but MRI is better to identify small tumors. The optic nerve gliomas appear as a fusiform shape due to the enlargement of the optic nerve (Figure 4C). Additionally, optic canal widening, eccentric optic nerve enlargement, and cystic degeneration can be seen in CT. MRI has benefits over CT scan in more precise identification of lesions and no risk of radiation exposure (25). Optic nerve sheath meningioma is one of the differentials which can be distinguished in MRI in T2W1. Meningioma, which arises from meninges, appears hypointense on T2WI of MRI, and post-contrast images show enhancement. In contrast, OPG is hyperintense in T2W1 and post-contrast shows variable enhancement (63).
Treatment options are observation, chemotherapy, and surgery. Gliomas, which are in a stable stage and slow-growing, can be kept in observation, and sometimes there is a spontaneous improvement.
It has been observed that tumors within the optic nerve during presentation usually remain in the optic nerve, and the risk of chiasm extension or metastasis is rare. Progressive tumors are treated with chemotherapy with vincristine and carboplatin. Radiotherapy, which is gamma knife radiosurgery in a fractionated way, is used in chemo-resistant cases. Though gamma knife radiosurgery can retard the progress or even reverse the progress, it has some potential side effects. In eyes with no visual potential but aggressive tumors, surgery is another therapeutic option. Surgery can be done through the orbital route by lateral orbitotomy or combinedly through transcranial and orbital approaches (25).
Vascular lesion
Capillary hemangioma
Capillary hemangioma is one of the commonest benign vascular tumors, and approximately 1 in 10 children is affected by capillary hemangioma of varying degrees of severity. In 90% of cases, these lesions are detected within 2 months of age, but they may present at birth. The tumor has a female predominance and premature or low birth weight infants are affected more frequently (64). Capillary hemangioma has many synonyms, such as strawberry hemangioma, strawberry nevus, capillary endothelioma, angioblastic hemangioma, hypertrophic hemangioma, and benign hemangioendothelioma.
Infantile hemangioma
Classic superficial hemangioma, which is known as strawberry nevus, appears as a reddish lesion, but it appears as bluish or purple in the subcutaneous type, and the deeper one is present as proptosis without any discoloration of the skin (65). Sometimes, a capillary hemangioma may associate with systemic disease such as (a) Kasabach-Merrit syndrome associated with thrombocytopenic purpura; consumptive coagulopathies associated with clotting factor defect; (b) microangiopathic hemolytic anemia (MAHA) where the erythrocytes are destroyed from coagulation or are sheared or fragmented by high pressure forcing them through the abnormally small vessels of the hemangioma; and (c) PHACES syndrome which is a cutaneous condition characterized by multiple congenital abnormalities like posterior fossa malformations hemangiomas, arterial anomalies, cardiac defects–eye abnormalities, sternal cleft, and supraumbilical raphe syndrome (64).
The natural history of capillary hemangioma shows the onset of a lesion a few weeks after birth and grows fast up to 1 year of age, which is termed the proliferative phase. The involutional phase follows the proliferative phase, which has intermediate stages of varying duration. Different studies have shown that most hemangiomas usually involute spontaneously and without any intervention the cosmetic result is good (66). Diagnosis is based on clinical examination, but imaging is needed if this lesion is associated with other syndromic diseases and helps to determine the extent of the lesion and the presence of any associated anomaly. Ultrasonography can identify well-circumscribed lesions, but for better resolution and deep lesions, MRI gives sensitive clues (25).
Observation with regular follow-up is enough for small capillary hemangioma, but bigger lesions, especially upper lid lesions, require treatment. Treatment is indicated to prevent amblyopia, in patients with a squint, proptosis, exposure keratopathy, and facial disfigurement. Different treatment modalities are tried to treat these hemangiomas, such as systemic and intralesional corticosteroids, radiotherapy, laser therapy, cyclophosphamide, vincristine, imiquimod, bleomycin, interferon alfa, radiotherapy, etc. All these modalities have various side effects and outcomes (64). Nowadays, most ophthalmologists and physicians are using propranolol either orally, in the local application as gel, or even as topical drops as first-line therapy to treat capillary hemangioma. The literature review showed very good outcomes with oral propranolol at a low dose of 1 mg/kg (Figures 5A, B) to a higher dose of 5 mg/kg body weight (67). Both the size and color of the hemangiomas change when treated with propranolol. Color changes within 1–3 days due to the vasoconstriction effect, which results from the decreased release of nitric oxide due to propranolol. The arrest of growth is the intermediate effect as a result of the blocking effect of different proangiogenic signals such as vascular endothelial growth factor, basic fibroblast growth factor, and matrix metalloproteinase. Tumor regression is the long-term result due to induction of apoptosis in proliferating endothelial cells (68).
Venous-lymphatic malformation (VLM)
It is a complex congenital lesion due to malformation of lymphatic and vascular structures during the embryogenic period. The ratio of composition of venous and lymphatic components varies according to the location of the lesion, such as superficial lesions containing more lymphatic components whereas venous components are more in deeper lesions. About 4% of orbital masses are venous-lymphatic lesions. Of the lesions, 43% are diagnosed before the age of 6 years and 60% before the age of 16 years (69).
Proptosis and ptosis are the most frequent presentations (Figure 6A). Proptosis may be fluctuating, but sometimes acute, painful proptosis occurs due to sudden enlargement or hemorrhage, and most of the time it is associated with infection of the upper respiratory tract (70). Chocolate-colored cysts of variable size may be found after hemorrhage. Other presentations are periocular swelling and facial disfigurement; extraocular motility restriction; ptosis; squint; amblyopia; astigmatism; and even compressive optic neuropathy, usually due to deeper lesions. Superficial lesions are detected earlier, and conjunctival lesions may be present with a small cyst filled with lymph. Growth of these tumors may be accelerated due to hormonal changes during puberty and pregnancy (71).

Figure 6. Venolymphatic malformation (lymphangioma). (A) Pretreatment presentation as proptosis and periocular swelling, (B) CT scan showing multiple microcyst, and (C) posttreatment picture with intralesional injection of bleomycin.
In imaging, these lesions appear as non-capsulated, irregular, lobulated lesions with ill-defined margins, which infiltrate different ocular structures involving intraconal, extraconal, preseptal, or post-septal portions of the orbit (Figure 6B). Both macrocyst and microcyst may be present, and the macrocyst within the lesion may measure up to 2 cm. In MRI, various components of the malformation are seen better, such as fluidic components (lymphatic or proteinaceous) on T1WI, non-hemorrhagic fluids on T2W, and blood on fat-suppressed T1 images. In VLM, fluid-fluid levels on imaging are supposed to be pathognomonic and are formed due to hemorrhage within the cysts. It is differentiated from hemangioma by flow voids, which are absent in VLM. Any orbital bony changes, like widening of orbital fissures or remodeling of orbital wall, are better delineated by CT scan. Literature showed that 70% of patients of VLMs may associate with intracranial vascular anomalies, and brain imaging should be performed concurrently (25).
Treatment occasionally needs multidisciplinary management, including interventional radiologists, craniofacial surgeons, and ophthalmologists. Treatment options are observation, sclerosing agents, medical treatment, and surgery. Medical management is not allowed in small children, and surgery is the most challenging. Different agents are used as serotherapeutic agents, such as sodium tetradecyl sulfate (3%), OK-432 (Picibanil), doxycycline, ethanol, pingyangmycin, and bleomycin (Figure 6C). These agents act by causing the significant local reaction. Initially, lesion size may increase due to internal hemorrhage, but it resolves over weeks (25).
Dermolipoma or lipodermoid
A lipodermoid or a dermoid is the most common type of orbital or epibulbar tumor in children (Figures 7A–D). These are choristomas containing epithelium-derived tissues (72). A conjunctival dermolipoma typically occurs near the lacrimal gland and lateral rectus muscle in the lateral canthus and superotemporal fornix. A dermolipoma, also known as a lipodermoid, is characterized by a deep fatty layer that gives it a yellow clinical appearance (73). On the surface, fine hairs are often visible. On histological examination, dermolipoma has layers of stratified squamous epithelium with a subepithelial stroma containing collagenous connective tissue and adipocytes. Furthermore, cartilage and glandular acini may be present in the stroma (74). It is essential to consider Goldenhar-Gorlin syndrome in cases of lipodermoids and other ocular abnormalities (75). Unlike many congenital syndromes, it occurs sporadically and cannot be inherited. However, autosomal dominant inheritance as well as chromosomal abnormalities have been reported (76).

Figure 7. Surface tumors. (A) Limbal dermoid, (B) conjunctival nevus, (C) conjunctival inclusion cyst, and (D) dermolipoma.
Small lesions generally are kept under observation, but larger lesions should undergo cautious debulking as excessive excision may result in diplopia, lacrimal gland injury, or ptosis (77). In most cases, surgery is done for cosmetic purposes. To avoid complications, it is important to isolate the lacrimal gland, levator, and Müller’s muscle complex, and lateral rectus muscle carefully (74).
Conjunctival melanocytic nevus
This lesion is benign in nature and usually appears in the first to the second decade of life. Initially, it remains superficial, and as time passes the nest of pigmented epithelium migrates to stromal layers. This lesion is more prominent in Caucasians (89%) than in Asians (5%) (78). Conjunctival nevi are mostly pigmented (84%) (Figure 7B) but may also be amelanotic or partially pigmented (16%).
Though the size, color, and location may vary, most nevi (72%) are found in the interpalpebral area near the limbus. Other sites of nevi are caruncle, semilunar folds, fornix, tarsus, and cornea. This nevus has characteristic clear cysts within the lesion which strongly support the diagnosis (79). These tumors sometimes also demonstrate feeder vessels (64%) and intrinsic vascularity (77%). There are some physiological causes for the increase in the size of the nevus, such as growing young children, puberty, pregnancy, and sun exposure (79). In most cases, it is benign, but malignant transformation may occur in less than 1% of cases (80).
Periodic observation is the mainstay of treatment. Observation is done annually with slit lamp measurement and serial photograph. A lesion is excised when there is any suspicion or cosmetic purpose, and it is advisable not to leave any residual lesion. The lesion should be suspected for malignant transformation if the lesion is greater than 10 mm in size; associated with engorged feeder vessels, prominent intrinsic vascularity with hemorrhage, lesions without cysts, uniformly dark lesion and corneal epithelial invasion >3 clock h and 3 mm from the limbus (10).
Conjunctival epithelial inclusion cyst
Conjunctival epithelial cysts or inclusion cysts may be spontaneous or posttraumatic. These cysts are smooth, translucent, and contain clear fluid (Figure 7C). The cyst may also contain turbid fluid, which is epithelial debris that looks like pseudohypopyon. These lesions usually remain asymptomatic and very slow-growing; sometimes auto-resolution may happen. A large cyst may cause lid swelling and even pseudoptosis when the upper lid is involved. Symptomatic or larger cysts need surgical excision (10).
Pyogenic granuloma
Pyogenic granuloma is a misnomer, and it is actually an exuberant granulation tissue. There are both vascular and fibrous tissue responses due to tissue injury by surgery, trauma, or inflammation, and are usually progressive and rapidly growing. These lesions may be pedunculated, ovoid, sessile, or mushroom-shaped, highly vascular, and with a fleshy red appearance. It arises from any part of the conjunctiva, limbus, and cornea (81). Histopathology shows the combination of granulation tissue with chronic inflammatory cells like lymphocytes and plasma cells, as well as scattered neutrophils and numerous small-caliber blood vessels. The topical steroid works well if the diagnosis is made earlier. Large, unsightly, symptomatic, and bleeding pyogenic granulomas are excised at the base, followed by cauterization or cryotherapy (10).
Discussion
A child can be affected by primary, secondary, or metastatic tumors. Primary tumors can be benign or malignant. The benign tumors are not always innocent, such as neurofibromatosis (NF 1), vascular tumors, and retinal capillary hemangioblastoma. In the case of NF 1, several surgical approaches may be needed for cosmetic reasons as well as vision. It may also be associated with optic nerve glioma and other retinal tumors. Retinal capillary hemangioblastoma is associated with Von Hipple Lindu’s disease, which may be life-threatening. Most of the time, patients are present when vision is lost in one eye. Early and prompt treatment can save residual vision, and systemic workup can save life also. The family members need proper counseling regarding the disease so that other members can be screened. Vascular tumors can also be vision threatening due to sudden hemorrhage and need urgent management with admission and prompt treatment to reduce the orbital volume, stabilize the blood vessel and plan for immediate surgical drainage. Some benign tumors are not aggressive and observation is enough, such as dermolipoma and conjunctival nevus. In regular practice, ophthalmologist often faces the demand of parents to remove these lesions. Counseling and regular follow-up can reduce unnecessary surgery with its complications, and surgery can be done in an indicated situation.
Childhood tumors are distinct forms of tumors that occur in adults. Many tumors are congenital and present earlier. As general people do not have any idea about childhood tumors, some life-threatening tumors that start in early life usually present with complications. Even some physicians are not so aware that tumors like retinoblastoma and rhabdomyosarcoma (Figure 8) can be present at birth or a few months after birth. Careful examination and proper referral can start prompt treatment and life can be saved. In the case of metastatic tumors, the choroid is the prime site for adults, but in the case of children, the orbit is the location of the first deposit. So any rapid onset proptosis should be taken care of by history evaluation, clinical examination of both ocular and systemic along with ancillary tests such as complete blood count and peripheral blood film. In suspected cases, a biopsy should be done.
Tumors have some age preference of presentation such as orbital tumors that are commonly found between birth to 4 years of age and common tumors are capillary hemangioma, dermoid cyst, lymphangioma, neuroblastoma, and teratoma; between 4–10 years lymphangioma, rhabdomyosarcoma, leukemic deposits, optic nerve glioma and dermoid cyst predominant; after 10 years dermoid, leukemic deposits are commonly found. Retinoblastoma is the most common intraocular tumor worldwide and can present from intrauterine life to old age, with a median age of presentation of 18 months. Retinoblastoma is also an example of a congenital, hereditary, genetic, and familial disease. There is a big list of differentials of white pupillary reflex, and these can be identified by precise history, ocular examination under general anesthesia, and imaging with B-Scan, CT scan, or MRI. Though B-Scan and CT scan show calcification in retinoblastoma, other different vascular tumors, and chronic ocular diseases may also cause calcification. Notably, any calcification under the age of 3 years should be suspected as a case of retinoblastoma (19). In suspected cases, serial monthly B-scan can be done for 2 to 3 months or enucleation is the choice of treatment where there is no visual potential. The parents need an actual understanding of the disease, information regarding treatment facilities, the outcome if being untreated, the genetic nature of the disease, and the availability of the treatment, which will increase the compliance of treatment.
Childhood tumor is a very wide spectrum of diseases. It is difficult to discuss all the tumors in one setup. The overall knowledge of an ophthalmologist can diagnose most childhood tumors. High suspicion is needed in atypical cases.
Conclusion
Childhood tumors are rare and different from those in adults. Proper knowledge and high suspicion of ophthalmologists along with counseling and timely referral can reduce morbidity and save life as well as vision.
References
1. Steliarova-Foucher E, Colombet M, Ries LA, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. (2017) 18:719–31.
2. World Health Organization [WHO].Cure All Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives. Geneva: World Health Organization (2021).
3. Coalition Against Childhood Cancer.Coalition Against Childhood Cancer, utilizing statistics from 2021. Philadelphia: Coalition Against Childhood Cancer (2021).
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics 2022. CA Cancer J. Clin. (2022) 72:7–33.
5. American Cancer Society.Cancer Facts & Figures 2022. Atlanta, GA: American Cancer Society (2022).
7. Kaliki S, Das A. ocular and periocular tumors in india: an eyesmart electronic medical record analysis of 9633 cases from a referral center. Middle East Afr J Ophthalmol. (2021) 27:199–203.
8. Juanarta P, Dahlan M, Rezano A. Characteristics of eye tumor in children diagnosed at the national eye center cicendo eye hospital. Althea Med J. (2017) 4:6–10.
9. Alkatan H Marek F, Elkhamary S. A demographics of pediatric orbital lesions: a tertiary eye center experience in Saudi Arabia. J Epidemiol Glob Health. (2019) 9, 3–10.
10. Honavar S, Manjandavida F. Tumors of the ocular surface: a review. Indian J Ophthalmol. (2015) 63:187–203. doi: 10.4103/0301-4738.156912
11. Broaddus E, Topham A, Singh A. Incidence of retinoblastoma in the USA: 1975-2004. Br J Ophthalmol. (2009) 93:21–3.
12. Jain M, Rojanaporn D, Chawla B, Sundar G, Gopal L, Khetan V. Retinoblastoma in Asia. Eye. (2019) 33:87–96.
13. Salim A, Wiknjosastro G, Danukusumo D, Barnas B, Zalud I. Fetal retinoblastoma. J Ultrasound Med. (1998) 17:717–20
14. Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. (2009) 93:1129–31.
15. Friend S, Bernards R, Rogelj S, Weinberg R, Rapaport J, Albert D, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. (1986) 323:643–6.
16. Rushlow D, Mol B, Kennett J, Yee S, Pajovic S, Thériault B, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. (2013) 14:327–34.
17. Shields J, Shields C, Parsons H. Differential diagnosis of retinoblastoma. Retina. (1991) 11:232–43.
18. Kaufman L, Mafee M, Song C. Retinoblastoma and simulating lesions. cRole of CT, MR imaging and use of Gd-DTPA contrast enhancement. Radiol Clin North Am. (1998) 36:1101–17.
20. Shields C, De Potter P, Himelstein B, Shields J, Meadows A, Maris J. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. (1996) 114:1330–8.
21. Friedman DL, Himelstein B, Shields CL, Shields JA, Needle M, Miller D, et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol. (2000) 18, 12–17.
22. Shield J, Shield C. Intraocular Tumor: A Text and Atlas. Philadelphia, PA: WB Saunders Company (1992).
23. Pradhan M, Ng Y, Strickland A George P, Raizis A, Warrington J, et al. Role of genetic testing in retinoblastoma management at a tertiary referral centre. Clin Experiment Ophthalmol. (2010) 38:231–6.
24. Tadepalli S, Shields C, Shields J, Honavar S. Intraocular medulloepithelioma – A review of clinical features, DICER 1 mutation, and management. Indian J Ophthalmol. (2019) 67:755–2.
25. Rao AA, John H, Naheedy J, Chen J, Shira L, Robbins S, Ramkumar H. A clinical update and radiologic review of pediatric orbital and ocular tumors. J Oncol. (2013), 2013:975908.
26. Sahm F, Jakobiec F, Meyer J, Schrimpf D, Eberhart C, Hovestadt V, et al. Somatic mutations of DICER1 and KMT2D are frequent in intraocular medulloepitheliomas. Genes Chromosomes Cancer. (2016) 55:418–27.
27. Sansgiri R, Wilson M, McCarville M, Helton K. Imaging features of medulloepithelioma: Report of four cases and review of the literature. Pediatr Radiol. (2013) 43:56
28. Martin K, Rossi V, Ferrucci S, Pian D Retinal astrocytic hamartoma. Optometry. (2010) 81:221–33.
29. Shields C, Douglass A, Higgins T, Samara W, Shields J. Retinal hemangiomas: understanding clinical features, imaging, and therapies. Retina Today. (2015) 10:61–67.
30. Rootman J. Diseases of the Orbit : A Multidisciplinary Approach. Philadelphia, PA: Lippincott Williams & Wilkins (2003).
31. Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. (2003) 48:39–57.
32. Turner JH, Richmon JD. Head and neck rhabdomyosarcoma: a critical analysis of population-based incidence and survival data. Otolaryngol Head Neck Surg. (2011) 145:967–73
33. Koopman JH, van der Heiden-van der Loo M, van Dijk MR, Bijlsma WR. Incidence of primary malignant orbital tumours in The Netherlands. Eye. (2011) 25:461–5.
34. Mafee MF, Pai E, Philip B. Rhabdomyosarcoma of the orbit. Evaluation with M.R. imaging and C.T. Radiol Clin. (1998) 36:1215–27
35. Frayer W, Enterline H. Embryonal rhabdomyosarcoma of the orbit in children and young adults. AMA Arch Ophthalmol. (1959) 62:203–10.
36. Kaliaperumal S, Tiroumal S, Rao V. Orbital rhabdomyosarcoma: a case series. Indian J Cancer. (2007) 44:104–107
37. Jurdy L, Merks J, Pieters B, Mourits M, Kloos R, Strackee S, et al. Orbital rhabdomyosarcomas: a review. Saudi J Ophthalmol. (2013) 27:167–75.
38. Terezakis SA, Wharam MD. Radiotherapy for rhabdomyosarcoma: indications and outcome. Clin Oncol. (2013) 25:27–35.
39. Sohaib SA, Moseley I, Wright JE. Orbital rhabdomyosarcoma-the radiological characteristics. Clin Radiol. (1998) 53:357–62
40. Conneely MF, Mafee MF. Orbital rhabdomyosarcoma and simulating lesions. Neuroimaging Clin N Am. (2005) 15:121–36.
42. Shields JA, Bakewell B, Augsburger JJ, Donoso LA, Bernardino V. Space-occupying orbital masses in children: a review of 250 consecutive biopsies. Ophthalmology. (1986) 93, 379–84.
43. Sherman R, Rootman J, Lapointe J. Orbital dermoids: clinical presentation and management. Br J Ophthalmol. (1984) 68:642–52.
44. Chawda S, Moseley I. Computed tomography of orbital dermoids: a 20-year review. Clin Radiol. (1999) 54:821–5.
45. Bonavolontà G, Tranfa F, de Conciliis C, Strianese D. Dermoid cysts: 16-year survey. Ophthal Plast Reconstr Surg. (1995) 11:187–92.
48. Nugent RA, Lapointe JS, Rootman J, Robertson WD, Graeb DA. Orbital dermoids, features on C.T. Radiology. (1987) 165: 475–8.
49. Shields J, Shields C. Orbital cysts of childhood–classification, clinical features, and management. Surv Ophthalmol. (2004) 49:281–99.
50. Reissis D, Pfaff M, Patel A, Steinbacher D. Craniofacial dermoid cysts: histological analysis and inter-site comparison. Yale J Biol Med. (2014) 87:349–57.
52. Filippi CG, Bos A, Nickerson JP, Salmela MB, Koski CJ, Cauley KA. Magnetic resonance diffusion tensor imaging (MRDTI) of the optic nerve and optic radiations at 3T in children with neurofibromatosis type I (NF-1). Pediatric Radiol. (2011) 42:168–74.
53. D’Ambrosio, J. Lyo J, Young R, Haque S, Karimi S. Common and unusual craniofacial manifestations of metastatic neuroblastoma. Neuroradiology. (2010) 52:549–53.
54. Yang W, Zhou Y, Zhao F, Mei Z, Li S, Xiang Y. Orbital neuroblastoma metastasis: a case report and literature review. Medicine. (2019) 98:e17038.
55. Mossé Y, Laudenslager M, Longo L, Cole K, Wood A, Attiyeh E, et al. Identification of ALK as the major familial neuroblastoma predisposition gene. Nature. (2008) 455:930–5.
56. Pflüger T, Schmid I, Coppenrath E, Weiss M. Modern nuclear medicine evaluation of neuroblastoma Q J Nucl Med Mol Imaging. (2010) 54, 389–400.
57. Ahmed S, Goel S, Khandwala M, Agrawal A, Chang B, Simmons IG. Neuroblastoma with orbital metastasis: ophthalmic presentation and role of ophthalmologists. Eye. (2006) 20:466–70.
58. Johnson E, Dean S, Sondel P. Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev Mol Med. (2007) 9:1–21.
59. Sharma T, Grewal J, Gupta S, Murray PI. Ophthalmic manifestations of acute leukaemias: the ophthalmologist’s role. Eye. 18, 663–672 (2004).
60. Russo V, Scott I, Querques G, Stella A, Barone A, Delle Noci N. Orbital and ocular manifestations of acute childhood leukemia: clinical and statistical analysis of 180 patients. Eur J Ophthalmol. (2008) 18:623.
61. Robert-Boire V, Rosca L, Samson Y, Ospina L, Perreault S. Clinical presentation and outcome of patients with optic pathway glioma. Pediatr Neurol. (2017) 75:55–60.
62. Huang M, Patel J, Patel B. Optic Nerve Glioma. Treasure Island, FL: StatPearls Publishing (2022).
63. Chung EM, Specht CS, Schroeder JW. From the archives of the AFIP: pediatric orbit tumors and tumor like lesions: neuroepithelial lesions of the ocular globe and optic nerve. Radiographics. (2007) 27:1159–86.
64. Roy S, Nuruddin M. Periocular capillary hemangioma treated with low dose oral propranolol - presentation and outcome of 30 patients. Arch Pathol Clin Res. (2021) 5:37–41.
65. Haik B, Jakobiec F, Ellsworth R, Jones I. Capillary hemangioma of the lids and orbit: an analysis of the clinical features and therapeutic results in 101 cases. Ophthalmology. (1979) 86:760–92.
66. Bang G M, Setabutr P. Periocular capillary hemangiomas: indications and options for treatment. Middle East Afr J Ophthalmol. (2010) 17:121–8.
67. Pope E, Krafchik B R, Macarthur C, Stempak D, Stephens D, Weinstein M, et al. Oral versus high-dose pulse corticosteroids for problematic infantile haemangiomas: a randomized, controlled trial. Pediatrics. (2007) 119:e1239–47.
68. Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. (2010) 163:269–74.
69. Nassiri N, Rootman J, Rootman D B, Goldberg RA. Orbital lymphaticovenous malformations: current and future treatments. Surv Ophthalmol. (2015) 60:383–405.
70. Chung EM, Smirniotopoulos JG, Specht CS, Schroeder JW, Cube R. From the archives of the AFIP: pediatric orbit tumors and tumorlike lesions: nonosseous lesions of the extraocular orbit. Radiographics. (2010) 27:1777–99
71. P. Dhellemmes, G. M. Breviere, C. Degrugillier-Chopinet, and M Vinchon. Vascular lesions of the orbit in children. Neurochirurgie (2010) 56, 271–280
73. Jakobiec F, Pineda R, Rivera R, Hsu-Winges C, Cherwek D. Epicorneal polypoidal lipodermoid: lack of association of central corneal lesions with goldenhar syndrome verified with a review of the literature. Surv Ophthalmol. (2010) 55:78–84.
74. McNab A, Wright J, Caswell A. Clinical features and surgical management of dermolipomas Australian and New Zealand. J Ophthalmol. (1990) 18:159–62.
75. Mansour A, Wang F, Henkind P, Goldberg R, Shprintzen R. Ocular findings in the facioauriculovertebral sequence (Goldenhar-Gorlin syndrome). Am J Ophthalmol. (1985) 100:555–9
76. Choong Y, Watts P, Little E, Beck L. Goldenhar and cri-du-chat syndromes: a contiguous gene deletion syndrome? J AAPOS. (2003) 7:226–7.
77. Kim E, Kim H, Kim Y, Woo K I, Lee H, Kim S T. Subconjunctival fat prolapse and dermolipoma of the orbit: differentiation on C.T. and M.R. Imaging AJNR. Am J Neuroradiol. (2011) 32:465–7 doi: 10.3174/ajnr.A2313
78. Cervantes G, Rodríguez A Jr, Leal A. Squamous cell carcinoma of the conjunctiva: Clinicopathological features in 287 cases. Can J Ophthalmol. (2002) 37:9. doi: P10.1016/S0008-4182(02)80093-X
79. Shields C, Belinsky I, Romanelli Gobbi M, Guzman J, Mazzuca D Jr, Green W, et al. Anterior segment optical coherence tomography of conjunctival nevus. Ophthalmology. (2011) 118:9.
80. Shields C, Demirci H, Karatza E, Shields J. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. (2004) 111:54





