1. Introduction
Meningiomas more than 30% of all brain tumors and are female-dominant tumors (1, 2). The recent estimation of the meningioma incidence rate in the population was 9.12 per 100,000 (2). The etiology of the tumor is not completely understood yet. Some data show the relationship between sex hormones and meningioma, but other data do not support this theory (2). Meningiomas are mostly benign tumors that grow slowly and have a good prognosis. But almost 20% of them will show recurrence after complete surgical resection (3). Meningiomas can become malignant and 0.1–1% of all these tumors metastasize to further destinations (4–8). The World Health Organization (WHO) classifies meningiomas according to their degree of anaplasia, necrosis, invasion of the brain, and amount of mitosis. Based on the WHO classification, there are three grades: benign (Grade I), atypical (Grade II), and malignant (Grade III) (3, 4). Meningiomas are often Grade I, benign, and cured after gross surgical resection. Grade II tumors represent almost 5–15%, and almost 1–3% are Grade III (1). Approximately 20% of meningiomas are Grade II (atypical) or Grade III (malignant), with a recurrence rate of up to 41% within 5 years (3). Higher-grade meningiomas show a low survival rate even following surgery and adjuvant treatment, and their 10-year survival rate is 50–80% (1). In a meta-analysis, Grade I meningiomas had a 29% of progression-free survival (PFS) rate during 6 months and this rate for Grade II/III tumors was 26% after systemic medical therapy (9). In some studies, meningiomas recur after surgical treatment at a rate of more than 20% (10). Rolandic meningioma has more relapse and disability than convex meningioma (11). Some patients will experience recurrence despite getting different treatment plans, including surgery, radiotherapy, and systemic therapy (12). Longer follow-up after treatment in patients has shown a 47% recurrence rate after 25 years, but we cannot determine which patient is going to experience recurrence based on WHO grading, which means we cannot determine which patient needs adjuvant therapies and which does not need it (13). Tumors with Simpson Grade IV should be watched for recurrence, and for recurrence control, we can use preoperative tumor embolization (14). Another risk factor in another study was the doubling time of the tumor. Tumors with a doubling time of fewer than 3 years had markedly higher MIB-1 SI, and those with a doubling time of less than 1 year had a higher risk of developing malignancy (4). In another study on spinal meningioma, younger patients had a recurrence rate significantly higher than older ones. Other risk factors include the size of the tumor (large tumor has more recurrence rate) and plaque lesions.
These recurrent tumors mostly had been misdiagnosed as nerve sheath tumors or lymphoma (15).
2. Diagnosis
Diagnosis of meningiomas is challenging because of the slow tumor progression dynamics (i.e., the tumor arises from the membranous layers surrounding the brain and spinal cord, not from neurons) (16, 17). Furthermore, meningioma symptoms are usually subtle and easily mistaken for other health conditions (e.g., migraine disorder and nausea) (18). Preliminary diagnosis is done via computerized tomography (CT) or magnetic resonance imaging (MRI) or using contrast dye (19). However, a biopsy with pathology analysis is necessary for a definitive diagnosis. Their initial management is often advocated with the presence of adverse symptoms and when growth is noted on serial imaging (20). This can include neurosurgery, radiation therapy, or a combination of both. However, although gross total resection (GTR) is an admirable goal, achieving GTR is very challenging, and surgeons often employ safer margins to avoid surgery-related comorbidity and adverse outcomes (e.g., skull base meningiomas) (20–22). Meningiomas recur over time, especially when patients receive less than GTR resections (13, 23). Management of recurrent meningioma is similar to primary treatment, mainly involving repeat surgery, but can involve adjuvant therapeutics, though the alternatives are less promising than surgical intervention (23).
As mentioned previously, meningioma growth (and regrowth) is monitored through serial imaging studies. Both CT and MRI are valuable imaging modalities used in the diagnosis of meningioma status, including during post-surgical surveillance for recurrence. Meningiomas are typically intracranial extra-axial masses; thus, their appearance is broad-based with dural attachment (22, 24). However, they can also extend in a wide sheet-like manner (25). Therefore, because of the broad range of visual appearances in imaging studies, different imaging modalities are used for their respective advantages. Furthermore, researchers have employed machine learning and artificial intelligence technologies to aid in meningioma diagnoses (primary and recurrent), which hold promising value in tumor diagnosis and recurrence (26, 27). In the following, we describe MRI and CT imaging for meningioma diagnoses and their respective implications.
2.1. MRI
MRIs provide a clearer, more detailed picture, often allowing for the delineation of changes, such as swelling, to be visualized and noted (19). In MRI, meningiomas are typically hypo-iso-intensive on T1 and iso-hyper-intensive on T2 (24, 28). In most cases, dura tail is present on post-contrast imaging, helping to identify meningioma from other neural pathologies (i.e., schwannoma) (29). Apparent diffusion coefficient (ADC) values of meningiomas vary in diffusion-weighted imaging (DWI) (30). Further, perfusion imaging usually reveals higher relative cerebral blood flow and blood volume in meningiomas (31). Another feature to help distinguish meningioma is the presence of a CSF cleft between the mass and the brain parenchyma (i.e., crescent on T2 imaging), although these clefts usually disappear in higher-grade meningiomas because of the invasion of the tumor (19). Most meningiomas (including benign and malignant meningiomas) have edema in adjacent brain tissues (32). A small subset of meningiomas display other features, including hemorrhage, tumor necrosis, cyst formation, and fatty infiltration (33). As the meningioma growth increases, the underlying brain is displaced inward (24).
2.2. CT
Conversely, CT combines X-ray images into a three-dimensional reconstruction, allowing abnormalities to be visualized, albeit at a lower resolution (24). Still, CTs are commonly used to measure tumor size for serial imaging surveillance as they are quicker to capture than MRI images. A clearly outlined lobular mass attached to a broad dural base is commonly visualized via contrast CT imaging. In comparison, on non-contrast CT imaging, meningiomas manifest as hyperdense extra-axial homogenous masses, which, respectively, enhance following contrast infusion (24, 29). Because of the slow growth characteristics of meningiomas, intratumor calcification is present. Moreover, dystrophic and metaplastic calcification can also occur, visualized as a hyperdense speckled mass (34). Bony changes (e.g., osteolysis and hyperostosis) associated with meningioma progression (or malignant Grade III meningiomas) are visualized well via CT and may also implicate osseous tumor invasion (Figure 1) (19).
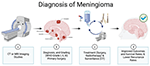
Figure 1. Flowchart of Meningioma Diagnosis. (a, b) Primary surgical intervention is performed for histopathological confirmation following primary diagnosis via imaging studies, CT, and/or MRI. (c, d) After primary treatment (surgery and/or therapeutics), follow-up serial imaging studies are performed to evaluate for meningioma recurrence. Surgery, chemotherapy, and other methods will be used to treat a recurrence of meningioma.
3. Location and recurrence
Atypical meningioma most commonly occurs in cerebral convexity 57% of the time. There are interhemispheric falx (12%), posterior fossa (10%), frontobasal (6%), parasagittal (4%), tentorial (4%), sphenoid wing (4%), and cavernous sinus (2%) (35). The non-skull-base meningiomas are usually atypical, while the skull-base meningiomas usually have lower Ki67-MIB1 values coinciding with the low-grade meningiomas (Figure 2) (36). When other factors are controlled that may increase high-grade meningioma risk, it has been shown that atypical and malignant skull base meningiomas are less likely to develop (37). Several reports have published a higher rate of atypia and malignancy in parasagittal and convexity meningiomas (38–41). This may be related to the different embryological origins of the dura in skull-base and non-skull-base locations. Neoplasia tends to emerge from the dura originating from discrete embryological tissue as in non-skull-base locations (42, 43). Moreover, the location of the meningioma may determine its surgical resectability and prognosis.

Figure 2. (A) Skull-base meningiomas are rarely atypical and have lower Ki67-MIB1 values coinciding with the low-grade meningiomas, which decrease their recurrence risk. (B) Non-skull-base meningiomas are usually atypical and have higher Ki67-MIB1 values coinciding with the high-grade meningiomas, which increases their recurrence risk.
4. Environmental factors determining recurrence
It is reported that head and neck irradiation is associated with an increased risk for meningioma incidence (44). Strojan reported a meningioma incidence risk of 0.53% at 5 years and 8.18% at 25 years after cranial irradiation (45). However, there is no evidence of the effect of radiotherapy on transformation to higher-grade tumors (46). A study by Phillips et al. found a correlation between traumatic brain injury and the incidence of meningioma (47). Hormonal factors may also be involved in the pathogenesis of meningioma explaining the high predominance of meningioma among females (48). During pregnancy, a meningioma may undergo aggressive behavior (49).
5. Surgical resectability and recurrence
For most cases of symptomatic meningioma, the primary treatment is surgical resection. Simpson grading is a well-known scale that guides the extent of meningioma resection, and there is a close relation between Simpson grading and the risk of recurrence (50). There is a high risk of early recurrence with high Simpson grades (III–V) (18, 51). With the more advanced neurosurgical imaging and navigation techniques, the authors believe that Simson grading has a limited value in predicting recurrence (52). Moreover, the extent of maximum resectability may be limited by the anatomical location of the tumor (53). Recurrence rates are correlated with Simpson grading in a variety of ways, ranging from a hazard ratio of 2.5 with each Simpson grade increase to a complete absence of any correlation (54, 55). Kira Marie et al. found that Simpson Grades III and IV correlate with recurrence only in convexity meningiomas, while they did not predict progression in falx/parasagittal meningiomas (52).
6. Radiotherapy and recurrence
6.1. Fractionated external beam radiotherapy
Radiotherapy is frequently used for symptomatic, primary, or recurrent Grade I meningiomas (20). For Grade II and III meningioma GTR/subtotal resection (SR), postoperative radiotherapy is recommended (56). Similar to surgical management of meningiomas, radiotherapeutic modalities are dependent on a variety of factors, including tumor and patient characteristics. In Matani et al.’s study, predictors of external beam radiotherapy (EBRT) are correlated with WHO Grade III disease severity among patients with meningioma treated with radiation (57). Nonetheless, for WHO Grade I tumors, both stereotactic radiosurgery (SRS) and EBRT are acceptable treatment options, based on NCCN guidelines (57). However, because of the infiltrative nature of meningiomas, treatment strategies for WHO Grade III tumors are usually more limited (EBRT alone or surgery with EBRT) (57).
In summary, fractionated radiosurgery or conventional fractionated EBRT is applicable when it is not possible to treat the tumor with a single fraction (20). This recommendation is supported by data only for recurrent, “high-risk” meningiomas in WHO Grade I tumors (20). When neurological symptoms are not present in patients with WHO Grade I meningiomas following incomplete resection, radiotherapy may not be necessary immediately after surgery (58). In fact, radiotherapy is prompted for patients with WHO Grade II meningiomas that have undergone a Simpson IV–V resection (59). For WHO Grade III, radical surgery is recommended, followed by fractionated EBRT (20).
6.2. Stereotactic radiosurgery
SRS is highly regarded as a minimally invasive method of treating recurrent meningiomas following surgery (60). Numerous publications have shown the safety and success of stereotactic radiosurgery for tumors in complex locations where surgical treatment is not feasible (61). In comparison with EBRT, SRS delivers lower radiation levels precisely without adversely affecting nearby tissues. There is still controversy as to whether SRS can improve tumor control as adjuvant therapy. Because SRS has been demonstrated to be effective in controlling small-to-moderate meningiomas, microsurgery combined with SRS results in high tumor control rates. The gold standard of meningioma surgical resection continues to be to minimize tumor effect, release vital neurovascular structures, and procure tissue samples for prognosis (60).
7. Adjuvant therapy and recurrence
Inoperable recurrent meningiomas are treated with hormones, chemotherapy, or targeted therapies.(62, 63).
7.1. Hormonal therapy
7.1.1. Estrogen and progesterone receptor antagonists
There is evidence that meningioma growth may be hormone-dependent both epidemiologically (predominance of females) and biochemically (70–80% express progesterone receptors, and 10–30% express estrogen receptors). Additionally, about 60% of meningiomas are stained for prolactin receptors (2, 64).
Meningiomas of advanced grade often lose their hormone receptor positivity. Because of this, recurrent benign meningiomas have been treated with hormonal therapies. There was no significant response to megestrol acetate, an oral progesterone agonist, in a small trial of nine patients (65).
Tamoxifen, an estrogen receptor antagonist, failed to show an advantage in inhibiting meningioma development in a Stage II review involving 19 patients with non-resectable meningiomas (66). This is likely due to the low frequency of meningioma-specific estrogen receptor expression in humans. On the other hand, progesterone receptor antagonists were alternatively viewed as potential treatment options in several studies because of the higher probability of progesterone receptor expression in meningiomas (66). However, a prospective, multi-center, randomized Phase III clinical trial with 180 participants failed to demonstrate any significant advantage of mifepristone (66). Furthermore, a recent study that looked at mifepristone in a pre-selected population with multiple meningiomas and a high progesterone receptor expression level found a sustained clinical and stabilization response. These studies support the use of mifepristone in future prospective clinical trials of populations and suggest that potential subgroups of meningioma patients can likely benefit from it (67).
7.1.2. Growth hormone and insulin-like growth factors I/II
Growth hormone (GH) has been studied in relation to meningiomas since initial observations that acromegaly increases the risk of meningiomas. IGF-1, which is synthesized by the liver in response to GH secretion, facilitates normal growth in combination with GH secretion. Meningiomas contain numerous GH receptors, which have been shown to decrease tumor growth in vitro when inhibited. Pegvisomant inhibits meningioma xenografts in mice by acting as a competitive antagonist of the GHR (68).
7.1.3. Somatostatin agents
Somatostatins are neuropeptides that are synthesized in the hypothalamus, regulating various physiological functions, such as neuromodulation, secretory inhibition, and cell proliferation (69).
Pasireotide, a long-acting intramuscular somatostatin analog, targets more somatostatin receptors than existing medications (67). Somatostatin receptor expression in meningiomas is known to be high (90%), particularly in the SST2A subtype, but the function of these receptors is still unknown (66).
The efficacy of somatostatin and somatostatin analogs on meningioma growth has been the subject of contradictory research in both human and experimental studies. Roughly one-third of the patients had a partial response. Another one-third of the patients had stable disease, and the remaining one-third had clinical progression (66, 69).
7.2. Chemotherapy
Molecular mechanisms, such as cellular differentiation, cell proliferation, angiogenesis, and apoptosis, are targeted by chemotherapeutic agents (70). Unfortunately, chemotherapy treatment for recurrent meningiomas is constrained by a lack of tumor models and pre-clinical research, as well as a lack of knowledge regarding the molecular pathogenesis of meningiomas (71). For the treatment of meningiomas, numerous conventional cytotoxic agents, such as temozolomide, hydroxyurea, imatinib, trabectedin, and irinotecan, have been investigated over time (71). However, clinical trial outcomes have typically been disappointing. The use of cytotoxic, chemotherapeutic agents in treating recurrent meningiomas is not recommended because of low efficacy rates (56). In general, conventional chemotherapy approaches are reserved as last-line, salvage therapies for patients if surgery and radiation become refractory (72). Retrospective and prospective studies investigated the use of compounds such as hydroxyurea, temozolomide, irinotecan, and trabectedin. Trabectedin is a chemotherapeutic drug that has accrued data from in vitro meningioma studies, but its mechanism of action is still not widely understood (69). Trabectedin is currently the only chemotherapeutic drug examined in a multicenter, randomized study for recurrent meningiomas of WHO Grades II/III (56). Multidrug chemotherapy trials are still limited, regardless of the aggressiveness, malignancy, or resistance of recurrent meningiomas to surgery and radiation. A chemotherapy regimen consisting of cyclophosphamide, vincristine, and adriamycin is generally administered as an adjuvant treatment for malignant meningiomas (63). There are no data available on response rates, duration of responses, or toxicities related to other investigational regimens. Hydroxyurea (HU) inhibits ribonucleotide reductase, a growth factor involved in meningioma growth, and induces apoptosis in these cells. Several studies have shown that HU has limited activity. The response rates are low, although some patients appear to have stabilized their disease (73, 74). Also, HU was shown to have modest toxicity, mostly manifested in the form of fatigue and anemia in patients with recurrent meningioma (75). A prospective multi-institution SWOG study (SWOG-S9811) was conducted to assess the effectiveness of HU in helping to treat meningiomas. However, the study ended early because of poor enrollment, and the initial results suggest moderate hematological toxicity and cytostatic activity (74).
7.3. Target therapy
Meningiomas and their molecular pathogenesis and molecular changes promoting meningioma growth are not well understood despite advances in molecular understanding. Several growth factors and pathways of signaling (i.e., PDGF, EGF, and VEGF) have been implicated (76, 77). PDGF drives cell proliferation both during normal development and in diseases, including cancer. Meningioma growth is believed to be influenced by PDGF (78, 79).
Over 60% of meningiomas express the EGF receptor (EGFR). Meningioma growth has been demonstrated to be stimulated by EGF and TGF-a in vitro, supporting the idea that autocrine/paracrine stimulation of EGFR might lead to the proliferation of meningiomas in humans. There has been an association between aggressive growth and TGF-a immunoreactivity in meningiomas (80).
For a subgroup of meningiomas, preoperative endovascular embolization treatment has become common because meningiomas are frequently highly vascular tumors (72). The main mediator of abnormal angiogenesis in these malignancies has been identified as the vascular endothelial growth factor (VEGF) (66). Differing levels of VEGF expression have been linked to both the WHO grade of meningiomas and the level of peritumoral cerebral edema (66). Researchers have studied humanized monoclonal antibodies directly inhibiting VEGF (e.g., bevacizumab, Avastin) as therapeutic agents aimed at stopping or reversing tumor-related angiogenesis (67). Phase II trials are necessary because there are a number of small-scale retrospective studies with conflicting results. Inhibition of VEGF receptors has been shown to have considerable anti-tumor effects, indicating that VEGF plays an important role in tumor angiogenesis (63, 69). Bevacizumab, an anti-VEGF antibody, has shown dramatic improvements in cancer survival (63). Meningiomas express VEGF and VEGFRs, which increase with the grade of the tumor (69). When compared to benign meningiomas, VEGF expression is two times higher in atypical meningiomas and ten times higher in malignant meningiomas (63). Further, VEGF increases the morbidity of these conditions by causing peritumoral edema (63, 66, 69). There has been little research on VEGF and VEGFR inhibitors in meningiomas. However, they have the potential to reduce edema and inhibit angiogenesis (66). In an antiangiogenic trial involving sunitinib, an inhibitor of tyrosine kinases with anti-VEGFR and anti-PDGF activity, 1 out of 28 patients demonstrated a halfway radiographic reaction. The remaining 17 patients displayed stable illness (69).
8. Other treatments
8.1. Interferon-alpha
Meningioma cell lines cultured in vitro are inhibited by recombinant Interferon-alpha (IFN-a). A retrospective case series study was performed on 35 cases of recurrent unresectable meningiomas that had previously undergone irradiation and were unresectable. Evidence suggests that IFN-a has antiproliferative, immunomodulatory, and antiangiogenic properties. In this trial, the most common toxicity was fatigue, which led to reduced dosage (seven patients), stimulant treatment (ten patients), and discontinuation of therapy early (three patients).
Neuroradiographic findings indicate that IFN-a is cytostatic as there were no radiographic responses but, instead, a stable disease state. The study’s primary objective of 40% PFS at 6 months was exceeded by 54%, indicating meaningful palliation. The median PFS was 7 months, despite the lack of radiographic responses (54 and 31%, respectively) (68).
8.2. Calcium channel blocker
Meningioma growth can be blocked by calcium channel antagonists such as verapamil, nifedipine, and diltiazem at clinically relevant doses, according to Ragel (81). Inhibition of calcium-dependent secondary messenger systems is believed to be the major mechanism by which calcium channel antagonists exert their antitumor effects (82).
Verapamil or diltiazem added to HU enhances in vitro and in vivo growth inhibition of meningiomas. However, seven patients are included in a clinical trial. Over the course of 14.5 months of follow-up, the patients failed to demonstrate significant radiographic response despite receiving 8.1 treatment cycles (74).
9. Conclusion
Meningiomas are commonly benign, growing slowly, and have a good prognosis. But there will be a poor prognosis once the tumor recurs. Meningiomas often recur after being removed. Histological factors such as MIB-1 and multiple mitoses may denote aggressive tumors. There is a high incidence of atypical meningioma in cerebral convexity. The importance of MRI follow-up in the early detection of recurrent meningiomas cannot be overstated as, in addition to neurological deficits, meningiomas may develop into atypical or malignant tumors with a greater risk of recurrence because of more mitotic activity and brain invasion. Women were more likely than men to suffer from recurrent meningiomas. There is a possibility that a meningioma will become aggressive during pregnancy. Recurrent meningiomas could only be treated with surgery and radiation therapy. There have been modest successes with chemotherapy. Since the introduction of targeted therapies and immunotherapies, our understanding of complex tumor genomics has grown considerably. Combining surgery with target therapy can increase patients’ survival.
Author contributions
BL-W and M-RH-S were the major contributors to the design of the study. M-RH-S, ZH, and SL were responsible for writing the first manuscript. M-RH-S and MK were responsible for revising the manuscript and validating the included studies. M-RH-S, MA, and MK contributed to editing graphs. BL-W and M-RH-S conceived the study and were in charge of the overall direction and planning. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
GTR, gross total resection; ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; STR, subtotal resection; EBRT, external beam radiotherapy; SRS, stereotactic radiosurgery; GH, growth hormone; HU, hydroxyurea; IFN-a, interferon-alpha.
References
1. Lu V, Luther E, Eichberg D, Morell A, Shah A, Komotar R, et al. The emerging relevance of H3K27 trimethylation loss in meningioma: a systematic review of recurrence and overall survival with meta-analysis. World Neurosurg. (2022) 163:87–95.e1. doi: 10.1016/j.wneu.2022.04.048
2. Hage M, Plesa O, Lemaire I, Raffin Sanson M. Estrogen and progesterone therapy and meningiomas. Endocrinology. (2022) 163:bqab259. doi: 10.1210/endocr/bqab259
3. Patel A, Wan Y, Al-Ouran R, Revelli J, Cardenas M, Oneissi M, et al. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc Natl Acad Sci U.S.A. (2019) 116:21715–26. doi: 10.1073/pnas.1912858116
4. Miwa Y, Sugawara T, Kobayashi D, Maehara T. Tumor doubling time as preoperative predictor of malignancy and recurrence in newly diagnosed meningioma. Neurosurg Rev. (2022) 45:3683–7. doi: 10.1007/s10143-022-01869-2
5. Vakil H, Tran L, Lewis G, Cykowski M, Butler E, Teh B. Biopsy proven metastatic meningioma: a case report and review of the literature. Rep Pract Oncol Radiother. (2019) 24:528–32. doi: 10.1016/j.rpor.2019.08.002
6. Wang M, Zhan R, Zhang C, Zhou Y. Multiple pulmonary metastases in recurrent intracranial meningioma: case report and literature review. J Int Med Res. (2016) 44:742–52. doi: 10.1177/0300060515618053
7. He L, Yu S, Wang L. Rapid recurrence and malignant transformation of a benign meningioma after pregnancy: a case report. Br J Neurosurg. (2020) 1–3. doi: 10.1080/02688697.2020.1817323
8. Zhao J, Liu J, Fang M, Luo C, Gu Z, Huang L. A rare case report of recurrent atypical meningioma with multiple metastases treated with anti-PD-1 and anti-VEGF therapy. BMC Neurol. (2022) 22:392. doi: 10.1186/s12883-022-02919-4
9. Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, et al. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. (2014) 16:829–40. doi: 10.1093/neuonc/not330
10. Gupta P, Shaikh S, Deopujari C, Shah N. Transnasal endoscopic surgery for suprasellar meningiomas. Neurol India. (2021) 69:630–5. doi: 10.4103/0028-3886.319224
11. Ottenhausen M, Rumalla K, Younus I, Minkowitz S, Tsiouris A, Schwartz T. Predictors of postoperative motor function in rolandic meningiomas. J Neurosurg. (2018) 130(4):1283–88. doi: 10.3171/2017.12.JNS172423
12. Saraf S, McCarthy B, Villano J. Update on meningiomas. Oncologist. (2011) 16:1604–13. doi: 10.1634/theoncologist.2011-0193
13. Pettersson-Segerlind J, Orrego A, Lönn S, Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. (2011) 76:564–71. doi: 10.1016/j.wneu.2011.05.015
14. Akimoto T, Yoshikawa H, Fushimi S, Takagi R, Nakamura T, Ohtake M, et al. Surgical complications and recurrence factors for asymptomatic meningiomas: a single-center retrospective study. Acta Neurochir (Wien). (2022) 1–9. doi: 10.1007/s00701-022-05420-6
15. Park B, Dougherty M, Noeller J, Nourski K, Gold C, Menezes A, et al. Spinal meningioma in adults: imaging characteristics, surgical outcomes, and risk factors for recurrence. World Neurosurg. (2022) 164:e852–60. doi: 10.1016/j.wneu.2022.05.054
16. Huttner H, Bergmann O, Salehpour M, El Cheikh R, Nakamura M, Tortora A, et al. Meningioma growth dynamics assessed by radiocarbon retrospective birth dating. EBioMedicine. (2018) 27:176–81. doi: 10.1016/j.ebiom.2017.12.020
17. Quddusi A, Virani Q, Shamim M. Factors affecting post-operative recurrence or growth of meningiomas, other than histological grade and extent of resection. J Pak Med Assoc. (2019) 69:1570–1.
18. Goldbrunner R, Minniti G, Preusser M, Jenkinson M, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. (2016) 17:e383–91. doi: 10.1016/S1470-2045(16)30321-7
19. Huang R, Bi W, Griffith B, Kaufmann T, la Fougère C, Schmidt N, et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro Oncol. (2019) 21(Suppl 1):i44–61. doi: 10.1093/neuonc/noy143
20. Goldbrunner R, Stavrinou P, Jenkinson M, Sahm F, Mawrin C, Weber D, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. (2021) 23:1821–34. doi: 10.1093/neuonc/noab150
21. Giammattei L, di Russo P, Starnoni D, Passeri T, Bruneau M, Meling T, et al. Petroclival meningiomas: update of current treatment and consensus by the EANS skull base section. Acta Neurochir (Wien). (2021) 163:1639–63. doi: 10.1007/s00701-021-04798-z
22. Lemée J, Corniola M, Da Broi M, Joswig H, Scheie D, Schaller K, et al. Extent of resection in meningioma: predictive factors and clinical implications. Sci Rep. (2019) 9:5944. doi: 10.1038/s41598-019-42451-z
23. Corniola M, Meling T. Management of recurrent meningiomas: state of the art and perspectives. Cancers (Basel). (2022) 14:3995. doi: 10.3390/cancers14163995
24. Buetow M, Buetow P, Smirniotopoulos J. Typical, atypical, and misleading features in meningioma. Radiographics. (1991) 11:1087–106. doi: 10.1148/radiographics.11.6.1749851
25. Toh C, Castillo M, Wong A, Wei K, Wong H, Ng S, et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol. (2008) 29:1630–5. doi: 10.3174/ajnr.A1170
26. Ugga L, Perillo T, Cuocolo R, Stanzione A, Romeo V, Green R, et al. Meningioma MRI radiomics and machine learning: systematic review, quality score assessment, and meta-analysis. Neuroradiology. (2021) 63:1293–304. doi: 10.1007/s00234-021-02668-0
27. Coroller T, Bi W, Huynh E, Abedalthagafi M, Aizer A, Greenwald N, et al. Radiographic prediction of meningioma grade by semantic and radiomic features. PLoS One. (2017) 12:e0187908. doi: 10.1371/journal.pone.0187908
28. Tamrazi B, Shiroishi M, Liu C. Advanced imaging of intracranial meningiomas. Neurosurg Clin N Am. (2016) 27:137–43. doi: 10.1016/j.nec.2015.11.004
29. O’Leary S, Adams W, Parrish R, Mukonoweshuro W. Atypical imaging appearances of intracranial meningiomas. Clin Radiol. (2007) 62:10–7. doi: 10.1016/j.crad.2006.09.009
30. Lusins J, Nakagawa H. Multiple meningiomas evaluated by computed tomography. Neurosurgery. (1981) 9:137–41. doi: 10.1227/00006123-198108000-00004
31. Hakyemez B, Yildirim N, Erdoǧan C, Kocaeli H, Korfali E, Parlak M. Meningiomas with conventional MRI findings resembling intraaxial tumors: can perfusion-weighted MRI be helpful in differentiation? Neuroradiology. (2006) 48:695–702. doi: 10.1007/s00234-006-0115-y
32. Lee K, Joo W, Rha H, Park H, Chough J, Hong Y, et al. Peritumoral brain edema in meningiomas: correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. (2008) 69:350–5; discussion 355. doi: 10.1016/j.surneu.2007.03.027
33. Russell E, George A, Kricheff I, Budzilovich G. Atypical computed tomography features of intracranial meningioma: radiological-pathological correlation in a series of 131 consecutive cases. Radiology. (1980) 135:673–82. doi: 10.1148/radiology.135.3.7384454
34. Sheporaitis L, Osborn A, Smirniotopoulos J, Clunie D, Howieson J, D’Agostino A. Intracranial meningioma. AJNR Am J Neuroradiol. (1992) 13:29–37.
35. Ressel A, Fichte S, Brodhun M, Rosahl S, Gerlach R. WHO grade of intracranial meningiomas differs with respect to patient’s age, location, tumor size and peritumoral edema. J Neurooncol. (2019) 145:277–86. doi: 10.1007/s11060-019-03293-x
36. Kane A, Sughrue M, Rutkowski M, Shangari G, Fang S, McDermott M, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. (2011) 117:1272–8. doi: 10.1002/cncr.25591
37. Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee J. World Health Organization grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery. (2007) 61:1194–8; discussion 1198. doi: 10.1227/01.neu.0000306097.38141.65
38. Sughrue M, Sanai N, Shangari G, Parsa A, Berger M, McDermott M. Outcome and survival following primary and repeat surgery for World Health Organization grade III meningiomas. J Neurosurg. (2010) 113:202–9. doi: 10.3171/2010.1.JNS091114
39. Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. (1986) 25:233–42. doi: 10.1016/0090-3019(86)90233-8
40. Engenhart-Cabillic R, Farhoud A, Sure U, Heinze S, Henzel M, Mennel H, et al. Clinicopathologic features of aggressive meningioma emphasizing the role of radiotherapy in treatment. Strahlenther Onkol. (2006) 182:641–6. doi: 10.1007/s00066-006-1555-3
41. Rosenberg L, Prayson R, Lee J, Reddy C, Chao S, Barnett G, et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. (2009) 74:427–32. doi: 10.1016/j.ijrobp.2008.08.018
42. Lee J, Sade B, Choi E, Golubic M, Prayson R. Meningothelioma as the predominant histological subtype of midline skull base and spinal meningioma. J Neurosurg. (2006) 105:60–4. doi: 10.3171/jns.2006.105.1.60
43. Kepes J. Presidential address: the histopathology of meningiomas. A reflection of origins and expected behavior? J Neuropathol Exp Neurol. (1986) 45:95–107.
44. Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S. Radiation-induced meningioma. Neurosurg Focus. (2008) 24:E7. doi: 10.3171/FOC/2008/24/5/E7
45. Strojan P, Popović M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. (2000) 48:65–73. doi: 10.1016/s0360-3016(00)00609-x
46. Niranjan A, Kondziolka D, Lunsford L. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin N Am. (2009) 42:717–29.
47. Phillips L, Koepsell T, van Belle G, Kukull W, Gehrels J, Longstreth W. History of head trauma and risk of intracranial meningioma: population-based case-control study. Neurology. (2002) 58:1849–52. doi: 10.1212/wnl.58.12.1849
48. Wiemels J, Wrensch M, Claus E. Epidemiology and etiology of meningioma. J Neurooncol. (2010) 99:307–14. doi: 10.1007/s11060-010-0386-3
49. Lusis E, Scheithauer B, Yachnis A, Fischer B, Chicoine M, Paulus W, et al. Meningiomas in pregnancy: a clinicopathologic study of 17 cases. Neurosurgery. (2012) 71:951–61. doi: 10.1227/NEU.0b013e31826adf65
50. Ildan F, Erman T, Göçer A, Tuna M, Baǧdatoǧlu H, Cetinalp E, et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base. (2007) 17:157–71. doi: 10.1055/s-2007-970554
51. Spille D, Hess K, Bormann E, Sauerland C, Brokinkel C, Warneke N, et al. Risk of tumor recurrence in intracranial meningiomas: comparative analyses of the predictive value of the postoperative tumor volume and the Simpson classification. J Neurosurg. (2020) 134:1764–71. doi: 10.3171/2020.4.JNS20412
52. Voß K, Spille D, Sauerland C, Suero Molina E, Brokinkel C, Paulus W, et al. The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J Neurooncol. (2017) 133:641–51. doi: 10.1007/s11060-017-2481-1
53. Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J Neurosurg. (2012) 117:121–8. doi: 10.3171/2012.3.JNS111945
54. Winther T, Torp S. Significance of the extent of resection in modern neurosurgical practice of World Health Organization grade i meningiomas. World Neurosurg. (2017) 99:104–10. doi: 10.1016/j.wneu.2016.11.034
55. Sughrue M, Kane A, Shangari G, Rutkowski M, McDermott M, Berger M, et al. The relevance of Simpson grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg. (2010) 113:1029–35. doi: 10.3171/2010.3.JNS091971
56. Brastianos P, Galanis E, Butowski N, Chan J, Dunn I, Goldbrunner R, et al. Advances in multidisciplinary therapy for meningiomas. Neuro Oncol. (2019) 21(Suppl. 1):i18–31. doi: 10.1093/neuonc/noy136
57. Matani H, Abel S, Yu A, Karlovits S, Wegner R. Trends in the use of radiation for meningioma across the United States. Radiat Oncol J. (2022) 40:29–36. doi: 10.3857/roj.2021.00563
58. Maggio I, Franceschi E, Tosoni A, Nunno V, Gatto L, Lodi R, et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. (2021) 10:CNS72. doi: 10.2217/cns-2021-0003
59. Mariniello G, de Divitiis O, Corvino S, Strianese D, Iuliano A, Bonavolontà G, et al. Recurrences of spheno-orbital meningiomas: risk factors and management. World Neurosurg. (2022) 161:e514–22. doi: 10.1016/j.wneu.2022.02.048
60. Williams B, Salvetti D, Starke R, Yen C, Sheehan J. Stereotactic radiosurgery for WHO II and III meningiomas: analysis of long-term clinical and radiographic outcomes. J Radiosurg SBRT. (2013) 2:183–91.
61. Ruge M, Tutunji J, Rueß D, Celik E, Baues C, Treuer H, et al. Stereotactic radiosurgery for treating meningiomas eligible for complete resection. Radiat Oncol. (2021) 16:22. doi: 10.1186/s13014-021-01748-y
62. Wen P, Quant E, Drappatz J, Beroukhim R, Norden A. Medical therapies for meningiomas. J Neurooncol. (2010) 99:365–78. doi: 10.1007/s11060-010-0349-8
63. Chamberlain M, Barnholtz-Sloan J. Medical treatment of recurrent meningiomas. Expert Rev Neurother. (2011) 11:1425–32. doi: 10.1586/ern.11.38
64. Maiuri F, Mariniello G, de Divitiis O, Esposito F, Guadagno E, Teodonno G, et al. Progesterone receptor expression in meningiomas: pathological and prognostic implications. Front Oncol. (2021) 11:611218. doi: 10.3389/fonc.2021.611218
65. Grunberg S, Weiss M. Lack of efficacy of megestrol acetate in the treatment of unresectable meningioma. J Neurooncol. (1990) 8:61–5. doi: 10.1007/BF00182088
66. Caruso G, Elbabaa S, Gonzalez-Lopez P, Barresi V, Passalacqua M, Caffo M. Innovative therapeutic strategies in the treatment of meningioma. Anticancer Res. (2015) 35:6391–400.
67. Al-Rashed M, Foshay K, Abedalthagafi M. Recent advances in meningioma immunogenetics. Front Oncol. (2020) 9:1472. doi: 10.3389/fonc.2019.01472
68. McCutcheon I, Flyvbjerg A, Hill H, Li J, Bennett W, Scarlett J, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. (2001) 94:487–92. doi: 10.3171/jns.2001.94.3.0487
69. Moazzam A, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus. (2013) 35:E18. doi: 10.3171/2013.10.FOCUS13341
70. Patel B, Desai R, Pugazenthi S, Butt O, Huang J, Kim A. Identification and management of aggressive meningiomas. Front Oncol. (2022) 12:851758. doi: 10.3389/fonc.2022.851758
71. Mair M, Berghoff A, Brastianos P, Preusser M. Emerging systemic treatment options in meningioma. J Neurooncol. (2022) 1–14. doi: 10.1007/s11060-022-04148-8
72. Wilson T, Huang L, Ramanathan D, Lopez-Gonzalez M, Pillai P, De Los Reyes K, et al. Review of atypical and anaplastic meningiomas: classification, molecular biology, and management. Front Oncol. (2020) 10:565582. doi: 10.3389/fonc.2020.565582
73. Swinnen L, Rankin C, Rushing E, Laura H, Damek D, Barger G. Phase II study of hydroxyurea for unresectable meningioma (Southwest Oncology Group S9811). J Clin Oncol. (2009) 27(15 Suppl.):2063–2063.
74. Karsy M, Hoang N, Barth T, Burt L, Dunson W, Gillespie D, et al. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: human and orthotopic xenograft studies. World Neurosurg. (2016) 86:210–9. doi: 10.1016/j.wneu.2015.09.060
75. Chamberlain M, Johnston S. Hydroxyurea for recurrent surgery and radiation refractory meningioma: a retrospective case series. J Neurooncol. (2011) 104:765–71. doi: 10.1007/s11060-011-0541-5
76. McMullen K, Stieber V. Meningioma: current treatment options and future directions. Curr Treat Opt Oncol. (2004) 5:499–509. doi: 10.1007/s11864-004-0038-y
77. Gupta V, Su Y, Samuelson C, Liebes L, Chamberlain M, Hofman F, et al. Irinotecan: a potential new chemotherapeutic agent for atypical or malignant meningiomas. J Neurosurg. (2007) 106:455–62. doi: 10.3171/jns.2007.106.3.455
78. Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. (2005) 64:1029–36. doi: 10.1097/01.jnen.0000189834.63951.81
79. Ragel B, Jensen R. Aberrant signaling pathways in meningiomas. J Neurooncol. (2010) 99:315–24. doi: 10.1007/s11060-010-0381-8
80. Raizer J, Abrey L, Lassman A, Chang S, Lamborn K, Kuhn J, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. (2010) 12:95–103. doi: 10.1093/neuonc/nop015
81. Ragel B, Couldwell W, Wurster R, Jensen R. Chronic suppressive therapy with calcium channel antagonists for refractory meningiomas. Neurosurg Focus. (2007) 23:E10. doi: 10.3171/FOC-07/10/E10
82. Ragel B, Jensen R. New approaches for the treatment of refractory meningiomas. Cancer Control. (2003) 10:148–58. doi: 10.1177/107327480301000206
83. Maier H, Öfner D, Hittmair A, Kitz K, Budka H. Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg. (1992) 77:616–23. doi: 10.3171/jns.1992.77.4.0616
84. Mahmood A, Caccamo D, Tomecek F, Malik G. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. (1993) 33:955–63. doi: 10.1227/00006123-199312000-00001