Introduction
Pathogenesis of intracranial aneurysms
A total of 3 to 5% of the general population is affected by cerebral aneurysms that are characterized by localized structural deterioration of the arterial wall along with the loss of the internal elastic lamina as well as the disruption of media. Cerebral aneurysms are vascular pathologies that arise from several risk factors ranging from genetic to acquired (1).
Familial preponderance has been shown by several studies to have a weak association with intracranial aneurysms; however, this has been linked to some genetic loci involved in proteins responsible for repair of the endothelium and maintaining the structural architecture of the vessel wall. Generally, aneurysms form at bifurcations, branch points, and regions of congenital hypoplasia and fenestrations (1). Hypertension is said to contribute significantly as it creates non-laminar blood flow, pulsatile pressure, and shear-stress-induced endothelial lining damage. These factors together weaken the internal elastic lamina, which is responsible for the maintenance of the structural integrity of the arterial wall. This leads to vessel distension and outpouching (1). A study on location, morphology, and presentation of intracranial aneurysms has been shown in the figures and tables below (2).
Risk of rupture
Aneurysm rupture is related to several risk factors including aneurysm size >5.0°mm, its location at the anterior cerebral artery including anterior communicating artery, posterior cerebral artery as well as posterior communicating artery, irregular shape/lobulation of the aneurysm, growth of the aneurysm >1 mm on serial scans, and gadolinium uptake in the aneurysm wall. The exact standard for estimation of the possibility of rupture of an aneurysm is still unclear.
Flow diverters
The concept behind flow diversion through flow diverters, a term coined by Lieber et al. (2004), stems from the deployment of a metallic stent in the parent artery that diverts blood inflow from the pouching aneurysm. Thus, creating less blood flow turbulence while maintaining adequate perfusion distally. As a result, the luminal space of an aneurysm gets obliterated from thrombus formation and creates a scaffold for neointima or endothelialization. However, this comes at a price. The placement of a metallic material inside the vessel would also make it susceptible to other complications such as stent stenosis or occlusion of the vessel. In this section, we will discuss the need for the usage of two antiplatelet drugs as a standard regimen for the endovascular placement of FD stents and its associated complications that could arise consequently.
Early experiments and trials
By the early 1990s, traditional endovascular treatment and aneurysmal clipping were challenging in treating some types of aneurysms (3). They conveyed higher risks for parent vessel occlusion in fusiform aneurysms or protrusion of packing materials from broad-neck aneurysms into parent vessels as well as aneurysm rupture and perforation (4–7). As a result, multiple experimental trials were carried out in animal models to find out the efficacy and safety of FDs in treating intracranial aneurysms (3, 7, 8). They had demonstrated the underlying mechanism of FDs by implementing metallic stents into the arteries of animal models.(3, 7, 8) Following the delivery of stent to the aneurysm neck by a catheter, it expands after the catheter sheath removal (9). Secondary to its self-expansion properties and its larger diameter than that of the parent artery, the deployed FD produces a mechanical outward force against the vessel wall stabilizing its location (7, 8). Once the metallic stent is fully deployed, the aneurysm has approximately one-third of its neck covered by the metal filaments of the FD (9). Despite having close to two thirds of the neck sparse entry pores for blood inflow, the reduced access to the aneurismal sac abruptly diminishes the vorticity of blood inflow and the associated turbulence effects. As a result, stasis of blood and thrombus formation ensues (3, 7).
Long term, the FDs form a scaffold for fibro-cellular migration and endothelialization forming a new circumferential layer of intima between and around the stent wires (3). The aneurysm orifice gets narrowed with time until it is almost completely sealed and separated from the parent artery (3, 7). Although the aforementioned studies had produced promising outcomes in vivo, the used stents were not primarily designed for intracranial aneurysm treatment and more studies about stent components and their effects on the blood flow hemodynamics were warranted (3, 7, 8). Accordingly, stent-only treatment was used in select patients such as rupture and failed or difficult-to-treat aneurysms (10–13). These case reports had shown incredible results and successful treatments (10–13). Concurrently, other experiments were being conducted to discover the optimal characteristics for FDs used to treat aneurysms (14). Researchers simulated the physiological conditions of the intravascular system and compared different stent parameters such as the filament thickness and porosity. These parameters significantly attributed to decreased blood flow into the sac and had varied results (15–18). The current FD is a cylindric tube of metallic filaments made mostly of cobalt-chromium and nickel-titanium to a smaller extent, braided with pores with metal surface area ranging from 25 to 35%(19).
Early clinical trials
In 2008, the Buenos Aires Experience was the first clinical study to publish the result of patients treated solely with the PED, a device that is used for pipeline embolization. The PED also happens to be the first designed flow diverter for intracranial aneurysms (20). They included 53 patients who had 63 intracranial aneurysms. Aneurysm types treated were confined to those with wide or giant neck aneurysms, non-saccular or recurrent aneurysms (20). Complete occlusion of the aneurysmal sacs was observed in 56, 93, and 95% of patients at the 3rd, 6th, and 12th months, respectively. Surprisingly, during the follow-up duration, patients had no ischemic or hemorrhagic complications (20). In-stent stenosis (ISS), which is a known complication of in vessel stenting, is attributed to the applied pressure of the strut against vessel walls leading to an inflammatory response and neointima formation, which can cause segmental hyperplasia and intraluminal narrowing (21). In this study, during the 3rd month follow-up period, they noted 3 patients suffering from mild ISS and 2 having moderate ISS whilst severe ISS was seen in 2 patients (20). However, by the 6th month follow-up, 1 mild ISS was resolved, and 1 moderate and 1 severe ISS had regressed to mild and moderate ISS, respectively (20). The drug regimen for all patients was 75 mg of clopidogrel and 325 mg of aspirin started at least 3 days pre-procedurally and maintained for at least 6 months (20).

Figure 1. Location of aneurysms: ICA: 86%, ACA: 1%, MCA: 1%, VA:8%, BA:4% (2).
Flow diverter and dual antiplatelet therapies (DAPT)
Flow diverter (FD) insertions into the blood vessels and manipulation of the catheter can induce platelet aggregations and thrombus formations mandating aggressive dual antiplatelet regimens (26). Clopidogrel with aspirin is the most widely used regimen, though some have introduced ticagrelor or prasugrel with aspirin instead (27, 28). The exposed metallic surfaces of stents and the process of endothelialization activate platelet aggregations leading to thromboembolism (27). Eventually, brain ischemia ensues resulting in catastrophic neurologic deficits and hemorrhagic transformation and possibly death (27, 28). Avoidance of such events and reduction in their occurrences have been maintained by using DAPT (27).

Figure 2. Morphology: Wide-neck saccular: 80%, Fusiform/dissecting: 20% (2).

Figure 3. Presentation: Asymptomatic: 64%, SAH from treated aneurysm >60 days: 1%, Recurrent aneurysm after coiling, SAC, or failed clipping: 15% (2).
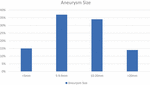
Figure 4. Aneurysm size: <5°mm: 15%, 5–9.9°mm: 37%, 10–20°mm: 34%, >20°mm: 14% (2).
Clopidogrel (Plavix) with aspirin
Clopidogrel is an antiplatelet prodrug metabolized in the liver to its active metabolites (28). The active drug exerts its effect by irreversibly inhibiting platelet P2Y12 adenosine diphosphate receptors (28). Although clopidogrel and aspirin have been used by many neuro-interventionists as the standard regimens prior and following FD installments, the duration and effective dosage of this regimen is widely debated (23–27). These regimens are generally administered at least 2°days pre-procedurally and maintained up to at least 6 months post stent placements (23–25). A systematic review and a combined analysis of 2002 patients who received clopidogrel and aspirin prior to and post aneurysmal stenting found that those who received clopidogrel for at least 6 months or more had a significantly lower risk of ischemic events compared to those treated for less than 6 months. Moreover, patients who were treated with higher doses of aspirin were found to have lower risks of stroke compared to lower aspirin doses (29). Despite no comparative studies between various clopidogrel doses among patients with FDs, a large-randomized control study in coronary stenting compared a 7°day regimen of clopidogrel with double the standard dose with a standard dose regimen and then maintained both groups on standard regimens following coronary stent interventions (30). They allocated four large groups into the following: (1) high dose of aspirin + standard dose of clopidogrel for 30 days; (2) low dose of aspirin + standard dose of clopidogrel for 30 days; (3) low dose of aspirin + double standard dose of clopidogrel for 7 days then followed by standard dose for the remaining 23 days; (4) high dose aspirin + similar regimen to group 3 (30). The group that received higher clopidogrel doses had significantly lower cardiovascular events and thrombosis in comparison to the standard dose irrespective of the aspirin dose. On the contrary, they found no significant differences in the efficacy and safety profile of high-dose aspirin in comparison with low-dose aspirin (30).

Figure 5. Common challenges encountered post flow diversion surgery (2).

Table 1. Risk factors of intracranial aneurysms (1).

Figure 6. Antiplatelet and antithrombotics in neurointerventional procedures (75).
DAPTs and the role of predictive value of platelet reactivity unit (PRU) assay in clopidogrel
The idea of using DAPTs emerged because up to 50% of the population have variable responses to clopidogrel based on genetic factors and lifestyles as well as presence of chronic diseases or concomitant use of other medications (31–33). Additionally, the use of clopidogrel and aspirin as DAPT was utilized in many of the previous FD trials and proved their efficacy in reducing thrombotic and hemorrhagic complications (29). Individual responsiveness to clopidogrel may be assessed by many means prior to interventions, but the most commonly used tool is VerifyNow P2Y12 assay (34). Unquestionably, some might debate the justification of using assessment tests to evaluate clopidogrel response whilst previous trials showed a low rate of complications without using these tools (22–25). However, on subsequent trials and with increased number of participants, researchers had enough data and were able to quantify clopidogrel response by using platelet reactivity unit (PRU) assay and its relation in predicting thromboembolic complications. A meta-analysis investigated the use of a PRU assay, specifically VerifyNow, as a predictive tool for thromboembolism and hemorrhage in clopidogrel hypo- and hyper-responders, respectively (35). The study involved over 1400 patients and showed that individuals with decreased response to clopidogrel were at a higher rate to develop thromboembolic events compared to normal clopidogrel responders. Moreover, individuals who conveyed hyperresponsivity to clopidogrel were at higher risks of developing hemorrhagic events. On the contrary, this test had no predictive value for hemorrhagic and thrombotic events in hypo-responsive and hyperresponsive states, respectively (35). Also, other factors justifying the use of PRU assay are the low cost of clopidogrel compared to other newer agents, and the need for adjusting clopidogrel doses in the event that other options are unavailable (36).

Table 3. Complication avoidance (76).
Ticagrelor (Brilinta) with aspirin
An active antiplatelet agent, ticagrelor, reversibly inhibits platelet P2Y12 adenosine diphosphate receptors (28). The introduction of ticagrelor with aspirin as DAPTs is not yet well established in neuro-endovascular interventions (37). However, ticagrelor is considered superior to and preferable to clopidogrel in cardio-interventions (38). There’s been a significant bend toward the use of ticagrelor or prasugrel over clopidogrel due to their efficacy in minimizing the risks of myocardial infarctions and ischemic strokes without significantly affecting the risk of bleeding (38). Recently, multiple retrospective studies have investigated ticagrelor as an alternative to clopidogrel with aspirin as DAPTs, and they found no significant differences in the outcomes and safety of ticagrelor with aspirin instead of clopidogrel and aspirin in intracranial unruptured aneurysmal treatment with FD stenting (37, 39–41). By contrast, a single meta-analysis and pooled analysis showed that patients treated with ticagrelor and aspirin had lower hemorrhagic incidences and better survival rates compared to clopidogrel with aspirin (42). The conflicting results might be attributed to the fact that the latter meta-analysis involved ruptured aneurysms in their study unlike the previous studies, which compared ticagrelor with clopidogrel in unruptured aneurysms (37, 39–42). This could indicate that using ticagrelor in ruptured aneurysms is superior to clopidogrel and further studies are warranted.
Prasugrel (Effient) with aspirin
Prasugrel is a prodrug that is converted into its active metabolites. The active form of prasugrel irreversibly inhibits platelet P2Y12 adenosine diphosphate receptors (28). Unlike clopidogrel, prasugrel is readily absorbed and efficiently converted into its active metabolites (28). Similarly, there is a significant trend in the utilization of prasugrel over clopidogrel in interventional cardiology due to its efficacious outcomes (43, 44). In interventional neurology, prasugrel has not been adequately investigated (45). However, a recent, large meta-analysis has analyzed the efficacy and safety profile of administering a loading dose of 20 mg and a maintenance dose of 5 mg in comparison to a standard dose of clopidogrel during and following neuro-interventional stenting. They found that the overall ischemic and thrombotic events were significantly lower in prasugrel (46). Additionally, the overall treatment-related complications were lower in prasugrel compared to clopidogrel but this did not reach statistical significance. Although previous studies showed a higher bleeding complication rate for patients treated with prasugrel compared to clopidogrel, the aforementioned meta-analysis showed the opposite but without reaching statistical significance (47). The fact that patients treated previously with prasugrel had received higher doses of prasugrel explains this (46, 47).
Single antiplatelet therapies (SAPT)
A meta-analysis study recently published has compared aspirin to ticagrelor or prasugrel in ruptured vs. unruptured aneurysms and coated vs. uncoated FDs (48). Their analysis suggested that aspirin as a monotherapy in FDs is associated with a relatively higher risk of ischemic events and the use of prasugrel or ticagrelor in ruptured aneurysms and coated FDs conveys potential promising outcomes (48). Another single retrospective study has introduced ticagrelor as a SAPT in 24 patients with 36 aneurysms, 14 of which were ruptured, who underwent FD stenting. Their results suggested the use of ticagrelor as a SAPT is effective in preventing ischemic events; however, in this the study population was small and larger studies are needed (49).
Dual antiplatelet therapy and emergent surgery for aneurysm rupture
For patients maintained on dual antiplatelet therapy after flow diversion, presenting with a ruptured aneurysm and requiring emergent surgery, it is imperative that procedure-related risk factors be adequately assessed. In the absence of specific reversal agents, general hemostatic measures should be undertaken for the management of bleeding, along with the cessation of antiplatelets or reversal of co-prescribed antithrombotics (50). Platelet function should also be assessed and platelet transfusion be considered accordingly (51). However, platelets need not be transfused earlier than 2°h after the last dose of aspirin and 12–24°h after the last dose of clopidogrel to avoid their inhibition by the circulating drugs or their active metabolites (50). Since platelet transfusion cannot fully reverse the effects of ticagrelor, a rapidly acting reversal agent, if developed, would be useful (51). There is a strong need for consensus guidelines on emergent neurosurgical procedures for patients maintained on dual antiplatelet therapy.
Complications
Despite the efficacy of FDs in the treatment of morphologically complex aneurysms for both on and off-label indications, these devices still come with a small yet significant and undeniable risk of peri-procedural and post-procedural complications. This mandates the need for a skilled operator for safe deployment of the device as well as close and careful post-procedural monitoring of the patient (52). The following complications are commonly related to the treatment in neuro-endovascular interventions according to a study by Fischer et al. (54): ischemic cerebrovascular accident in about 1.9% of cases, transient ischemic attack in 2.0% of cases, intracranial hemorrhage in 2.3%, subarachnoid hemorrhage in 1.1% and PED migrations in 0.3% of cases, side branch occlusion in 2.3%, and groin/peritoneal hematomas in 1.2% (53). Deep parenchymal bleeds into areas supplied by the parent vessel are very unlikely (54).
Ischemia and stroke
Although the ischemia risk associated with FDs is low, it is still a source of concern; however, the use of DAPTs has highly contributed to the low incidence of ischemia (23–25). Multiple meta-analyses and systemic reviews have shown nearly similar outcomes with slight variabilities, which might be attributed to multiple reasons; first, individual responses to clopidogrel and other antiplatelets; secondly, locations of the aneurysms; thirdly, type of stent material used; and finally, the duration of treatment (55). A meta-analysis done by F. Cagnazzo et al. analyzed various factors following the treatment of unruptured distal anterior circulation aneurysms with flow diverters. The study showed that the overall incidence of ischemic events of all locations was 9.6% and the highest ischemic occurrence located in MCA territory with 14.6% incidence rate compared to other locations. Ischemic incidence due to discontinuation of APTs prior to the required duration was around 2.8%. In regard to the posterior circulation, a meta- analysis has shown that FD installments in the non-saccular posterior circulation aneurysms are associated with as much as 25% of ischemic events, which could be explained by the fact that aneurysmal locations or morphology played significant roles in this relative high rate (56–58).
Hemorrhage and aneurysm ruptures
Hemorrhage risks have relatively increased with FD treatment due to long-term consumption of DAPTs and periprocedural manipulation of catheters, which can result in perforation or aneurysmal ruptures (59). Fortunately, hemorrhagic events are very low among FD-treated patients and rarely lead to permanent disability or death (59). Three meta-analyses have analyzed the hemorrhage rate based on the locations of aneurysms during and following the FD treatment and there was a very low risk ranging from a 2 to 3% risk (56, 57, 59). Similarly, aneurysmal ruptures have very low risks in FD-treated individuals either on short or long terms (56, 57, 59).
In-stent stenosis (ISS)
In-stent stenosis (ISS) is defined as any presence of intraluminal narrowing in the parent vessel at any point along the stent (60). ISS can be attributed to the neointima growth and/or aggregation of thrombus over the struts. There are multiple classifications for determining the severity of ISS (60). The NASCET criterion is generally used to calculate the percentage of the narrowed segment as well as defining the mild, moderate, and severe ISS as <50%, 50–70%, and 70–99%, respectively (61). Conversely, most authors used <50%, 50–75%, and >75% as mild, moderate, and severe ISS, respectively (60). Furthermore, John et al. attributes the first 25% narrowing to the neointima growth (62). ISS is a very rare complication after the FD insertion and its clinical manifestations among patients with ISS is very rare as well (60). A study compared ISS at their institution with their result of a systemic review and found the incidence of ISS among both groups to be 8.8% and 6.4, respectively. Interestingly, they noticed that none of their patients had clinical complaints expect one patient who had a subclinical stroke (60). Moreover, 25% of the patients with ISS had stable ISS and 16.6% had resolved and around 8% had dramatically improved on subsequent imaging and long-term follow-up (60). By contrast, another single study has determined the incidence of ISS among their patients to be as high as 29% of 205 treated candidates; however, no patient had significant clinical consequences (63). This might be explained by the fact that the latter had utilized p64 FDs, nitinol compared to the first study, PED, cobalt alloy.
In-stent thrombosis
One of the major complications associated with flow diversion surgery is in-stent thrombosis, some of which are peri-procedural, and others are delayed, but less common. This happens despite the usage of aspirin or clopidogrel as antiplatelet therapy, and can be fatal. These patients are often identified during follow-up with evidence of minimal residual flow into the aneurysm fundus. It has been reported in some studies that multi-layered PED construction meant for improving the efficacy of flow diversion raises the risk of parent artery thrombosis. In some cases of thrombosis, the underlying reason was non-compliance with antiplatelet therapy by the patients. Hence the effectiveness of antiplatelets should be monitored as this could be useful in reducing the cases of delayed parent vessel thrombosis. It is recommended that patients be placed on adequate antiplatelet therapy before the FDS procedure and post-treatment for at least several months to prevent parent vessel thrombosis.
Raymond-Roy Occlusion classification system and Modified Raymond-Roy classification are the commonly used classification system for assessing the success of the flow diversion procedure. The mechanism of intra-aneurysmal thrombus formation, as a means of aneurysm exclusion, has been evidenced by detailed fluid dynamics analyses of post-PED-treated vessels. FDs result in reductions in aneurysmal inflow and wall shear stress that provide an avenue for promoting parent vessel remodeling. Aneurysms treated with FDs with shorter time to occlusion have been shown to exhibit different hemodynamic than those with longer occlusion times, with significantly lesser mean aneurysmal inflow rate, velocity, and shear rate in the shorter time group (64).
A study found intra-aneurysmal thrombus in all thirteen aneurysms that were initially treated with FDs and subsequently ruptured. It was thought that persisting intraluminal thrombus triggered autolytic vessel wall destruction, leading to aneurysmal rupture. However, only two cases in the study were histopathologically assessed to have exhibited unorganized thrombi (64).
Though in-stent restenosis remains a recurrent problem in the coronary vasculature, the occurrence following FD placement is not high. Therefore, it is hypothesized that the endothelium may provide the source of a potential biomarker that, if found, may predict successful aneurysm occlusion. Many unique molecules have been studied in recent years, including C-reactive protein, eosinophilic cationic protein, matrix metalloproteinases, and lectins. An efficient biomarker must have certain characteristics, which include cost effectiveness, high specificity, and high sensitivity (64).
Aneurysmal occlusion
One of the main goals for treating aneurysms with FDs is achieving complete obliteration of the aneurysmal sac and separation from the parent vessel (7). A complete or near-complete aneurysmal occlusion can be defined as (grade C or D on O’Kelly-Marotta grade scale) or (class I or II on Raymond-Roy scale) (65, 66). Complete or near-complete occlusion rates are controversial and many studies have shown variable results. Furthermore, Kiyofuji et al. (58) has identified the overall complete occlusion rates to be 52% on the long term and the aneurysmal size as a single independent factor for a complete occlusion. However, other studies have shown significantly higher occlusion rates with preferable trends toward dissecting aneurysms compared to fusiform (57, 67, 68).
Aneurysm rupture after flow diversion
An important post-procedural complication, though rare, is delayed rupture of the aneurysm. Mechanical pressure and intra-aneurysmal thrombus formation promoting local inflammation and autolysis have been postulated as some of the risk factors for delayed aneurysm rupture. Other factors that might have a role include large and giant aneurysms, symptomatic aneurysms, saccular aneurysms having an aspect ratio of greater than 1.6, delayed migration of the FD into the aneurysmal sac, and mechanical injury by the FD (69). Furthermore, some flow diversions have insufficient flow resulting in abnormal intra-aneurysmal flow patterns, which can lead to a sudden change in the pattern of flow leading to increased strain to areas that were not previously exposed to stress, playing a significant role in delayed rupture of the parent vessel (70). A study reported instability in flow pattern and higher energy loss compared with pretreatment as being important hemodynamic factors playing a role in delayed aneurysm rupture (71).
A study showed that the incidence of aneurysm rupture after flow diversion surgery was 4.0%. Most of the cases were those with giant intracranial aneurysms. The main goal of flow diverters is to allow room for thrombosis within the aneurysm leading to treatment; however, it remains controversial as some believe that this thrombosis and resulting inflammation may worsen the prognosis of the aneurysm (71).
Nearly 1/5th of ruptures after flow diversion occur in previously ruptured aneurysms (72).
Some computational fluid dynamic studies have shown that changes to flow dynamics post-flow diversion result in increased intra-aneurysmal pressure leading to rupture. Other studies have linked this to the formation of a thrombus and not changes in flow dynamics; as the thrombus serves to generate several proteases with lytic function, hence breaking down the inner wall of the artery resulting in rupture. Some advice that large aneurysms be managed with simultaneous coiling and flow diversion, as this method protects the dome of the intracranial aneurysm. This again remains controversial; as it is found that associated coiling doesn’t totally prevent rupture since 20% of coiled aneurysms still rupture, despite combination with flow diversion. Some recommend high-density packing as it is seen to provide a more protective mechanism against delayed rupture. Ultimately, rupture of an aneurysm after flow diversion therapy is a complex, multifactorial event, and preclinical trials would be necessary to evaluate the mechanical and biological protective impact of associated coiling (72).
Giant aneurysms often require the deployment of multiple concurrent flow diverters to attain complete vessel occlusion (72). Deployment of these flow diverters could increase procedure duration, and also potentiate activation of platelet and eventually result in a more substantial hemodynamic instability than would deployment of a single flow diversion. In addition, hemodynamic alterations following therapy for a giant aneurysm may be more severe than when treating a lesser-sized aneurysm (72).
The deployment of a flow diverter after treatment of a giant aneurysm could result in the immediate loss of a wide capacitance chamber (i.e., the giant aneurysm) eventually leading to cerebral hyperperfusion distal to the aneurysm. Similar hemodynamic changes are also observed following surgical clipping of larger cerebral artery aneurysms (72). Rouchaud et al. (72) found that 76.6% of the delayed ruptures occurred within the first 30 days post-procedure. In the patients presenting with delayed rupture, the prognosis was poor, with more than 80% experiencing demise or neurological deficits. Giant aneurysms accounted for nearly half of the ruptures. More than 80% of these aneurysms were unruptured initially. Less than 20% of the delayed ruptured aneurysms had a previous or current history of coiling. Delayed intraparenchymal hemorrhages (DIPHs) were ipsilateral to the treated aneurysm in more than 80% of cases. A total of 86.0% of the DIPH were found within 30°days post-flow diversion surgery. One-fifth of DIPHs occurred in patients with giant aneurysms. In addition, 80% of patients who experience rupture after flow diversion often experience it in the first 30 days postop (72).
A prospective, multicenter study by the name of SAFE assessed the safety and efficacy of FRED (a flow diverting device) in the treatment of aneurysms (73). The study was unique in that it wasn’t restricted to the predefined indications for FD, but extended to off-label uses as well, such as small and distal aneurysms. However, FDs in ruptured aneurysms and those located in the posterior circulation were excluded from this study. A total of 103 patients were included in the study out of which 98 patients were successfully treated. Thromboembolic complications occurred in 5 patients, intraoperative rupture in 2 patients, delayed aneurysm rupture occurred in one patient, and delayed hematoma in one patient. The reported delayed aneurysm rupture occurred in a patient with a large supraclinoid aneurysm that was treated with FRED and no coils and was reported 21 days after the procedure with associated morbidity at 6 months (mRS = 5, coma). The rate of delayed intracranial hemorrhage reported in this study (1.0%) is similar to what was observed in another prospective, multicenter study by the name of PUFS (1.9%). The lack of a clearly proven pathology accounting for delayed rupture makes further studies focusing on this aspect of FD a necessity (73).
The clinical consequences of delayed aneurysm rupture range from acute intracranial bleeding i.e., subarachnoid hemorrhage or intracerebral hemorrhage to carotid-cavernous fistula, depending on the location of the aneurysm (69). Intracranial hemorrhage is a significantly fatal event with the general state of patient’s health deteriorating so rapidly that most patients often can get no further treatment, succumbing to inevitable demise. Even with the most aggressive management, the documented survival rate is low (69).
Patients presenting with carotid-cavernous fistula have been documented to have a more favorable outcome. Although there is insufficient evidence to support an optimal course of treatment, treatment options mentioned in literature include transvenous embolization, operative methods such as surgical ligation, and as a last resort, parent artery sacrifice. In a few cases, use of further FDs concomitantly with transvenous embolization has also been documented (69).
Strategies to avoid delayed aneurysm rupture
According to a meta-analysis, aneurysms of >2 cm were likelier than smaller aneurysms to rupture after treatment with FDs. Similarly, they reported improved clinical as well as angiographic outcomes in aneurysms of <7°mm, suggesting the possibility of flow diversion being more favorable and effective in smaller aneurysms. Adjunctive coiling might be worthwhile though, if large aneurysms are to be treated with FDs. Another reported strategy to treat large aneurysms with FDs, minimizing the risk of rupture is a staged treatment strategy of initial coiling followed by FD placement (74). A study by Rouchaud et al. (72) suggests high-density packing as possibly being more protective against delayed rupture.
Conclusion
The use of FDs has revolutionized the treatments and approaches to intracranial aneurysms, especially those hard to treat. However, this procedure is not risk free and might lead to detrimental side effects. Thankfully, the use of DAPTs has made these complications very rare. Currently, Clopidogrel and aspirin are the standard regimens used in neuro-endovascular interventions. On the other hand, in cardiological interventions, Prasugrel and Ticagrelor have been shown to be superior to Clopidogrel in terms of the thromboembolic consequences. Although in terms of neuro-endovascular interventions, the use of Prasugrel and Ticagrelor have not well been established and need further studies, some small studies have shown promising outcomes.
References
1. Etminan N, Dörfler A, Steinmetz H. Unruptured intracranial aneurysms- pathogenesis and individualized management. Dtsch Arztebl Int. (2020) 117:235–42. doi: 10.3238/arztebl.2020.0235
2. Taschner CA, Stracke CP, Dorn F, Kadziolka KB, Kreiser K, Solymosi L., et al. Derivo embolization device in the treatment of unruptured intracranial aneurysms: a prospective multicenter study. J Neurointerv Surg. (2021) 13:541–6. doi: 10.1136/neurintsurg-2020-016303
3. Wakhloo A, Schellhammer F, de Vries J, Haberstroh J, Schumacher M. Self-expanding and balloon-expandable stents in the treatment of carotid aneurysms: an experimental study in a canine model. AJNR Am J Neuroradiol. (1994) 15:493–502.
4. Halbach V, Higashida R, Hieshima G, Dowd C, Barnwell S, Edwards M, et al. Aneurysms of the petrous portion of the internal carotid artery: results of treatment with endovascular or surgical occlusion. AJNR Am J Neuroradiol. (1990) 11:253–7.
5. Graves V, Strother C, Partington C, Rappe A. Flow dynamics of lateral carotid artery aneurysms and their effects on coils and balloons: an experimental study in dogs. AJNR Am J Neuroradiol. (1992) 13:189–96.
6. Malisch T, Guglielmi G, Viñuela F, Duckwiler G, Gobin Y, Martin N, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg. (1997) 87:176–83. doi: 10.3171/jns.1997.87.2.0176
7. Geremia G, Haklin M, Brennecke L. Embolization of experimentally created aneurysms with intravascular stent devices. AJNR Am J Neuroradiol. (1994) 15:1223–31.
8. Turjman F, Acevedo G, Moll T, Duquesnel J, Eloy R, Sindou M. Treatment of experimental carotid aneurysms by endoprosthesis implantation: preliminary report. Neurol Res. (1993) 15:181–4. doi: 10.1080/01616412.1993.11740132
9. Kallmes D, Ding Y, Dai D, Kadirvel R, Lewis D, Cloft H. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. (2007) 38:2346–52. doi: 10.1161/STROKEAHA.106.479576
10. Marks M, Dake M, Steinberg G, Norbash A, Lane B. Stent placement for arterial and venous cerebrovascular disease: preliminary experience. Radiology. (1994) 191:441–6. doi: 10.1148/radiology.191.2.8153318
11. Benndorf G, Herbon U, Sollmann W, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. AJNR Am J Neuroradiol. (2001) 22:1844–8.
12. Lanzino G, Wakhloo A, Fessler R, Hartney M, Guterman L, Hopkins L. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. (1999) 91:538–46. doi: 10.3171/jns.1999.91.4.0538
13. Horowitz M, Miller G, Meyer Y, Carstens G, Purdy P. Use of intravascular stents in the treatment of internal carotid and extracranial vertebral artery pseudoaneurysms. AJNR Am J Neuroradiol. (1996) 17:693–6.
14. Fiorella D, Woo H, Albuquerque F, Nelson P. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. (2008) 62:1115–20;discussion 1120–1. doi: 10.1227/01.neu.0000325873.44881.6e
15. Wakhloo A, Tio F, Lieber B, Schellhammer F, Graf M, Hopkins L. Self-expanding nitinol stents in canine vertebral arteries: hemodynamics and tissue response. AJNR Am J Neuroradiol. (1995) 16:1043–51.
16. Lieber B, Livescu V, Hopkins L, Wakhloo A. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Ann Biomed Eng. (2002) 30:768–77. doi: 10.1114/1.1495867
17. Aenis M, Stancampiano A, Wakhloo A, Lieber B. Modeling of flow in a straight stented and nonstented side wall aneurysm model. J Biomech Eng. (1997) 119:206–12. doi: 10.1115/1.2796081
18. Rhee K, Han M, Cha S. Changes of flow characteristics by stenting in aneurysm models: influence of aneurysm geometry and stent porosity. Ann Biomed Eng. (2002) 30:894–904. doi: 10.1114/1.1500406
19. Dmytriw A, Phan K, Moore J, Pereira V, Krings T, Thomas A. On flow diversion: the changing landscape of intracerebral aneurysm management. AJNR Am J Neuroradiol. (2019) 40:591–600. doi: 10.3174/ajnr.A6006
20. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna H, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. (2009) 64:632–42; discussion 642–3;quizN6. doi: 10.1227/01.NEU.0000339109.98070.65
21. Kornowski R, Hong M, Tio F, Bramwell O, Wu H, Leon M. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. (1998) 31:224–30. doi: 10.1016/s0735-1097(97)00450-6
22. Hanel R, Cortez G, Lopes D, Nelson P, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device (PREMIER study): 3-year results with the application of a flow diverter specific occlusion classification. J Neurointerv Surg. (2023) 15:248–54. doi: 10.1136/neurintsurg-2021-018501
23. Briganti F, Leone G, Cirillo L, de Divitiis O, Solari D, Cappabianca P. Postprocedural, midterm, and long-term results of cerebral aneurysms treated with flow-diverter devices: 7-year experience at a single center. Neurosurg Focus. (2017) 42:E3. doi: 10.3171/2017.3.FOCUS1732
24. Fujii T, Teranishi K, Yatomi K, Suzuki K, Mitome-Mishima Y, Kondo A, et al. Long-term follow-up results after flow diverter therapy using the pipeline embolization device for large or giant unruptured internal carotid artery aneurysms: single-center retrospective analysis in the Japanese population. Neurol Med Chir. (2022) 62:19–27. doi: 10.2176/nmc.oa.2021-0203
25. Becske T, Potts M, Shapiro M, Kallmes D, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. (2017) 127:81–8. doi: 10.3171/2015.6.JNS15311
26. Saber H, Kherallah R, Hadied M, Kazemlou S, Chamiraju P, Narayanan S. Antiplatelet therapy and the risk of ischemic and hemorrhagic complications associated with Pipeline embolization of cerebral aneurysms: a systematic review and pooled analysis. J Neurointerv Surg. (2019) 11:362–6. doi: 10.1136/neurintsurg-2018-014082
27. Adeeb N, Griessenauer C, Foreman P, Moore J, Shallwani H, Motiei-Langroudi R, et al. Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke. (2017) 48:1322–30. doi: 10.1161/STROKEAHA.116.015308
28. Bonney P, Yim B, Brinjikji W, Walcott B. Pharmacogenomic considerations for antiplatelet agents: the era of precision medicine in stroke prevention and neurointerventional practice. Cold Spring Harb Mol Case Stud. (2019) 5:a003731. doi: 10.1101/mcs.a003731
29. Leung A, Li V, de Villiers L, Hattingh L. A comparison of antiplatelet therapy during the peri- and post-operative periods following flow-diverting stent insertion for unruptured intracranial aneurysms: a systematic review. Interv Neuroradiol. (2023): doi: 10.1177/15910199221148551 [Epub ahead of print].
30. Mehta S, Tanguay J, Eikelboom J, Jolly S, Joyner C, Granger C, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. (2010) 376:1233–43. doi: 10.1016/S0140-6736(10)61088-4
31. Oliphant C, Trevarrow B, Dobesh P. Clopidogrel response variability: review of the literature and practical considerations. J Pharm Pract. (2016) 29:26–34. doi: 10.1177/0897190015615900
32. Angiolillo D, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass T, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. (2007) 49:1505–16. doi: 10.1016/j.jacc.2006.11.044
33. Ho P, Maddox T, Wang L, Fihn S, Jesse R, Peterson E, et al. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. (2009) 301:937–44. doi: 10.1001/jama.2009.261
34. Jeong Y, Bliden K, Antonino M, Park K, Tantry U, Gurbel P. Usefulness of the verifynow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am Heart J. (2012) 164:35–42. doi: 10.1016/j.ahj.2012.03.022
35. Ajadi E, Kabir S, Cook A, Grupke S, Alhajeri A, Fraser J. Predictive value of platelet reactivity unit (PRU) value for thrombotic and hemorrhagic events during flow diversion procedures: a meta-analysis. J Neurointerv Surg. (2019) 11:1123–8. doi: 10.1136/neurintsurg-2019-014765
36. Moore J, Adeeb N, Shallwani H, Gupta R, Patel A, Griessenauer C, et al. A multicenter cohort comparison study of the safety, efficacy, and cost of ticagrelor compared to clopidogrel in aneurysm flow diverter procedures. Neurosurgery. (2017) 81:665–71. doi: 10.1093/neuros/nyx079
37. Papaxanthos J, Cagnazzo F, Collemiche F, Barreau X, Radu R, Gariel F, et al. Ticagrelor versus clopidogrel dual antiplatelet therapy for unruptured intracranial aneurysms treated with flowdiverter. J Neuroradiol. (2023) 50:346–51. doi: 10.1016/j.neurad.2022.11.010
38. Wallentin L, Becker R, Budaj A, Cannon C, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
39. Narata A, Amelot A, Bibi R, Herbreteau D, Angoulvant D, Gruel Y, et al. Dual antiplatelet therapy combining aspirin and ticagrelor for intracranial stenting procedures: a retrospective single center study of 154 consecutive patients with unruptured aneurysms. Neurosurgery. (2019) 84:77–83. doi: 10.1093/neuros/nyy002
40. Park K, Ozaki T, Kostynskyy A, Kortman H, Hilario A, Nicholson P, et al. Ticagrelor versus clopidogrel in the dual antiplatelet regimen for intracranial stenting or flow-diverter treatment for unruptured cerebral aneurysms: a single-center cohort study. AJNR Am J Neuroradiol. (2021) 42:1638–44. doi: 10.3174/ajnr.A7216
41. Soize S, Foussier C, Manceau P, Litré C, Backchine S, Gawlitza M, et al. Comparison of two preventive dual antiplatelet regimens for unruptured intracranial aneurysm embolization with flow diverter/disrupter: a matched-cohort study comparing clopidogrel with ticagrelor. J Neuroradiol. (2019) 46:378–83. doi: 10.1016/j.neurad.2019.01.094
42. Podlasek A, Al Sultan A, Assis Z, Kashani N, Goyal M, Almekhlafi M. Outcome of intracranial flow diversion according to the antiplatelet regimen used: a systematic review and meta-analysis. J Neurointerv Surg. (2020) 12:148–55. doi: 10.1136/neurintsurg-2019-014996
43. Wiviott S. Intensity of antiplatelet therapy in patients with acute coronary syndromes and percutaneous coronary intervention: the promise of prasugrel? Cardiol Clin. (2008) 26:629–37. doi: 10.1016/j.ccl.2008.07.003
44. Koshy A, Giustino G, Sartori S, Hooda A, Feng Y, Snyder C, et al. Ticagrelor or prasugrel versus clopidogrel in patients undergoing percutaneous coronary intervention for chronic coronary syndromes. EuroIntervention (2023) 18:1244–53. doi: 10.4244/EIJ-D-22-00654
45. Camargo L, Lima P, Janot K, Maldonado I. Safety of oral P2Y12 inhibitors in interventional neuroradiology: current status and perspectives. AJNR Am J Neuroradiol. (2021) 42:2119–26. doi: 10.3174/ajnr.A7303
46. Cagnazzo F, Perrini P, Lefevre P, Gascou G, Dargazanli C, Riquelme C, et al. Comparison of prasugrel and clopidogrel used as antiplatelet medication for endovascular treatment of unruptured intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol. (2019) 40:681–6. doi: 10.3174/ajnr.A6004
47. Akbari S, Reynolds M, Kadkhodayan Y, Cross D, Moran C. Hemorrhagic complications after prasugrel (Effient) therapy for vascular neurointerventional procedures. J Neurointerv Surg. (2013) 5:337–43. doi: 10.1136/neurintsurg-2012-010334
48. Senol Y, Orscelik A, Ghozy S, Kobeissi H, Arul S, Bilgin C, et al. The safety profile of single antiplatelet therapy with flow diverters: systematic review and meta-analysis. Interv Neuroradiol. (2023): doi: 10.1177/15910199231168669 [Epub ahead of print].
49. Mohammaden M, English S, Stapleton C, Khedr E, Shoyb A, Hegazy A, et al. Safety and efficacy of ticagrelor as single antiplatelet therapy in prevention of thromboembolic complications associated with the Pipeline Embolization Device (PED): multicenter experience. J Neurointerv Surg. (2020) 12:1113–6. doi: 10.1136/neurintsurg-2020-015978
50. Singh A, Singh R, Kerai S. Dual antiplatelet therapy challenges in emergent noncardiac surgeries. Med J. (2021) 14:230–3. doi: 10.4103/mjdrdypu.mjdrdypu_252_20
51. Tonetti D, Jankowitz B, Gross B. Antiplatelet therapy in flow diversion. Neurosurgery. (2020) 86(Suppl 1):S47–52. doi: 10.1093/neuros/nyz391
52. Al-Mufti F, Amuluru K, Cohen E, Patel V, El-Ghanem M, Wajswol E, et al. Rescue therapy for procedural complications associated with deployment of flow-diverting devices in cerebral aneurysms. Oper Neurosurg. (2018) 15:624–33. doi: 10.1093/ons/opy020
53. Oishi H, Teranishi K, Nonaka S, Yamamoto M, Arai H. Symptomatic very delayed parent artery occlusion after flow diversion stent embolization. Neurol Med Chir. (2016) 56:350–3. doi: 10.2176/nmc.cr.2016-0053
54. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. (2012) 54:369–82. doi: 10.1007/s00234-011-0948-x
55. Briganti F, Leone G, Marseglia M, Mariniello G, Caranci F, Brunetti A, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: a systematic review. Neuroradiol J. (2015) 28:365–75. doi: 10.1177/1971400915602803
56. Cagnazzo F, Perrini P, Dargazanli C, Lefevre P, Gascou G, Morganti R, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. AJNR Am J Neuroradiol. (2019) 40:687–93. doi: 10.3174/ajnr.A6002
57. Cagnazzo F, Lefevre P, Derraz I, Dargazanli C, Gascou G, di Carlo D, et al. Flow-diversion treatment for unruptured nonsaccular intracranial aneurysms of the posterior and distal anterior circulation: a meta-analysis. AJNR Am J Neuroradiol. (2020) 41:134–9. doi: 10.3174/ajnr.A6352
58. Kiyofuji S, Graffeo C, Perry A, Murad M, Flemming K, Lanzino G, et al. Meta-analysis of treatment outcomes of posterior circulation non-saccular aneurysms by flow diverters. J Neurointerv Surg. (2018) 10:493–9. doi: 10.1136/neurintsurg-2017-013312
59. Cagnazzo F, Mantilla D, Lefevre P, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with flow-diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol. (2017) 38:2289–94. doi: 10.3174/ajnr.A5388
60. Ravindran K, Salem M, Enriquez-Marulanda A, Alturki A, Moore J, Thomas A, et al. Quantitative assessment of in-stent stenosis after pipeline embolization device treatment of intracranial aneurysms: a single-institution series and systematic review. World Neurosurg. (2018) 120:e1031–40. doi: 10.1016/j.wneu.2018.08.225
61. North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
62. John S, Bain M, Hui F, Hussain M, Masaryk T, Rasmussen P, et al. Long-term follow-up of in-stent stenosis after pipeline flow diversion treatment of intracranial aneurysms. Neurosurgery. (2016) 78:862–7. doi: 10.1227/NEU.0000000000001146
63. Aguilar Pérez M, Bhogal P, Henkes E, Ganslandt O, Bäzner H, Henkes H. In-stent stenosis after p64 flow diverter treatment. Clin Neuroradiol. (2018) 28:563–8. doi: 10.1007/s00062-017-0591-y
64. Ravindran K, Salem M, Alturki A, Thomas A, Ogilvy C, Moore J. Endothelialization following flow diversion for intracranial aneurysms: a systematic review. AJNR Am J Neuroradiol. (2019) 40:295–301. doi: 10.3174/ajnr.A5955
65. O’kelly C, Krings T, Fiorella D, Marotta T. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. (2010) 16:133–7. doi: 10.1177/159101991001600204
66. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. (2001) 32:1998–2004. doi: 10.1161/hs0901.095600
67. Lin N, Lanzino G, Lopes D, Arthur A, Ogilvy C, Ecker R, et al. Treatment of distal anterior circulation aneurysms with the pipeline embolization device: a US multicenter experience. Neurosurgery. (2016) 79:14–22. doi: 10.1227/NEU.0000000000001117
68. Griessenauer C, Ogilvy C, Adeeb N, Dmytriw A, Foreman P, Shallwani H, et al. Pipeline embolization of posterior circulation aneurysms: a multicenter study of 131 aneurysms. J Neurosurg. (2018) 130:923–35. doi: 10.3171/2017.9.JNS171376
69. Hou K, Li G, Lv X, Xu B, Xu K, Yu J. Delayed rupture of intracranial aneurysms after placement of intra-luminal flow diverter. Neuroradiol J. (2020) 33:451–64. doi: 10.1177/1971400920953299
70. Kulcsár Z, Houdart E, Bonafé A, Parker G, Millar J, Goddard A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. (2011) 32:20–5. doi: 10.3174/ajnr.A2370
71. Li W, Tian Z, Zhu W, Zhang Y, Wang K, Zhang Y, et al. Hemodynamic analysis of postoperative rupture of unruptured intracranial aneurysms after placement of flow-diverting stents: a matched case-control study. AJNR Am J Neuroradiol. (2019) 40:1916–23. doi: 10.3174/ajnr.A6256
72. Rouchaud A, Brinjikji W, Lanzino G, Cloft H, Kadirvel R, Kallmes D. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview. Neuroradiology. (2016) 58:171–7. doi: 10.1007/s00234-015-1615-4
73. Pierot L, Spelle L, Berge J, Januel A, Herbreteau D, Aggour M, et al. Feasibility, complications, morbidity, and mortality results at 6 months for aneurysm treatment with the flow re-direction endoluminal device: report of SAFE study. J Neurointerv Surg. (2018) 10:765–70. doi: 10.1136/neurintsurg-2017-013559
74. Madaelil T, Moran C, Cross D, Kansagra A. Flow Diversion in ruptured intracranial aneurysms: a meta-analysis. AJNR Am J Neuroradiol. (2017) 38:590–5. doi: 10.3174/ajnr.A5030
75. Schirmer C, Bulsara K, Al-Mufti F, Haranhalli N, Thibault L, Hetts S, et al. Antiplatelets and antithrombotics in neurointerventional procedures: guideline update. J Neurointerv Surg. (2023) 15:1155–62. doi: 10.1136/jnis-2022-019844
76. Al-Mufti F, Cohen E, Amuluru K, Patel V, El-Ghanem M, Nuoman R, et al. Bailout strategies and complications associated with the use of flow-diverting stents for treating intracranial aneurysms. Interv Neurol. (2020) 8:38–54. doi: 10.1159/000489016
© The Author(s). 2024 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
