Introduction
Carnitine extensively used as a dietary supplement in the food and feed sectors. Pure carnitine is extremely hygroscopic, making handling and storage difficult. As a result, providing and using L-carnitine in salt form has various benefits.
There are two different types of carnitine. It’s important to distinguish between levocarnitine (L-carnitine) and D-L carnitine (marketed as “vitamin B T”). The body only utilizes the L-form of carnitine to cure severe carnitine shortages. The D-L form hinders levocarnitine production and does not aid the body in using fat. Levocarnitine is a quaternary ammonium molecule used to treat carnitine deficiency or to increase pancreatic and gastric secretions in hyperlipoproteinemia.
For the proper operation of the heart, brain, muscles, and many other bodily processes, L-carnitine is essential. It functions as a fat burner or aids in efficient fat metabolism. It aids in the breakdown of fat and its conversion into energy, aiding in weight management for a lean and toned figure.
L-carnitine L-tartrate is prepared in tablets, capsules, and various preparation forms for oral consumption. The preparations have better storage properties, longer stability, and less hygroscopicity than those prepared using free L-carnitine. L-carnitine promotes healthy development in children, aids in treating haemorrhagic shock and fatty liver, among other medical conditions, and promotes heart and blood vessel health.
Objectives of the study
The objectives of the study are listed as follows:
1. To enhance the flavor of the effervescent formulation
2. To improve L-carnitine stability
3. To reduce the moisture content of L-carnitine
4. To reduce the negative effects of L-carnitine
5. To boost the effectiveness of L-carnitine
6. Conduct compatibility studies with other excipients
Advantages
L-carnitine is mostly used for weight loss, brain function, depression, diabetes, and metabolic health issues.
It is used as a weight gainer in both liquid and solid dosage forms, including tablets with sustained release, capsules, and effervescent tablets.
Disadvantages
Rare side effects include muscle weakness, discomfort, diarrhoea, vomiting, and nausea.
Method development for the stability of L-carnitine
The inner salt of the compound is mixed with the least quantity of water required to produce a paste or semiliquid slurry at room temperature as a technique for producing a stable, non-hygroscopic L(-)-carnitine salt. Furthermore, L(-)-carnitine acid fumarate (1:1) and L(-)-carnitine L(+)-tartrate may be produced using this procedure (2:1). Further, 10–30% of the total slurry is used to make a semiliquid slurry or paste.
Fumaric acid and L-carnitine inner salt should be equilibrated at room temperature, or you can combine fumaric acid and L-tartaric acid by adding half as much fumaric acid as needed. Then, thoroughly blend the liquid that results from these two reactions. The reaction mixture from step (b) is solidified or dehydrated by allowing it to stand in the open air with a relative humidity of no more than 50%, or the solidification reaction mixture may be accelerated by drying and optionally grinding to produce L-carnitine salt as a granulate or powder product (Table 1).
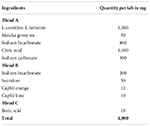
Table 1. Formulation of L-carnitine effervescent tablet 1 containing citric acid as an acid source Formulation 1.
There are several types of dryers used for drying, including continuous dryers, batch dryers, turboratory dryers, tray dryers, direct-heat rotary dryers, drum dryers, belt dryers, spray dryers, and fluid-bed dryers.
Citric acid is used in this formulation together with the active substances L-carnitine and matcha green tea. According to further analysis of the compatibility study with active components and excipients, citric acid was discovered to be quite hygroscopic in nature and determined to be incompatible after 1 month of stability testing with L-carnitine and matcha green tea. Orange and lime flavors, which are also used in this formulation, give the drink a slightly acidic taste before it becomes sweet.
But people do not like the flavor of oranges. Therefore, the formulation must be altered to have an exceptional flavor, a sufficient disintegration time, and ingredient compatibility. This way, it is ensured that the active components and excipients do not interact.
L-carnitine with matcha green tea effervescent powder processing technique
The formulations were made with sodium bicarbonate, citric acid, and sodium carbonate as release disintegrants. There are several flavors and sucralose used as a sweetener. Also used as a lubricant is 1% boric acid. Some formulations employ color to create the appearance of change.
All the excipients and components were weighed precisely. It is possible to process drugs with excipients without the use of granulation and associated unit activities. It is possible to process formulation ingredients by only blending them in a blender.
Table 2 shows the following:

Table 2. Formulation of L-carnitine effervescent tablet 1 containing malic acid as an acid source (1).
1. Preparation of Blend A
Add L-carnitine, matcha green tea, sodium bicarbonate, citric acid, and sodium carbonate and thoroughly mix. The mixture should be run through sieves #20 or #24.
2. Preparation of Blend B
Mix thoroughly before adding sodium bicarbonate, sucrose, and the capSil orange and capSil lime flavors. The mixture should be run through filter #60.
3. Preparation of Blend C
Boric acid is prepared by passing it through sieve No. 60. In blend B, combine it.
4. Preparation of Blend D
Add the FCF color in sunset yellow and the #100 pass-through sieve.
In a plastic bag, combine the entire mixture by shaking it to create an even mixture.
Conditions for the procedure
A low relative humidity environment with a maximum of 25% or less and moderate to cold temperatures (about 25°C or 77°F) are required to prevent sticking granules.
Formulation 2
Malic acid is employed in this formulation along with the active substances L-carnitine and matcha green tea. Malic was discovered to be compatible with L-carnitine and matcha green tea after further analysing the compatibility study with active components and excipients. Additionally, the use of flavors like strawberry and pineapple in this formulation results in a slightly acidic taste that is followed by a sweet sensation.
It is observed that 90% of individuals enjoy the flavor of strawberry and pineapple. As a result, the formulation has a wonderful flavor and is compatible with the ingredients. As a result, the active substances and excipients do not interact.
As a result, when comparing formulations 1 and 2, it is discovered that formulation 2 has a superior flavor and appearance. Sunset yellow was the color chosen, and it has no impact on the formulation. The color is Food and Drug Administration (FDA)-approved, and both the color and the formulation as a whole have no negative effects on people.
Matcha green tea is an antioxidant, so it gives the body and mind a new feeling, making L-carnitine and matcha green tea a good combination for patients with fat problems and body gain.
Consequently, it supports active muscular and brain activity.
Characterization of powder blend
The characteristics of the powder mix for effervescent tablets included angle of repose, bulk density, tapped density, Carr’s index, and medication content. An angle of repose less than 35° and Carr’s index values under 21, which indicate good to fair flowability and compressibility, were present in all of the batches’ granules. Hausner’s ratio was under 1.25 for each of the batches, suggesting satisfactory flow properties. The drug content exceeded 90% for all granules of different formulations.
Determination of effervescent solution pH
One tablet is dissolved in 200 ml of filtered water at 20 ± 1 °C for the duration of the dissolve time, and then the pH of the solution is measured using a pH meter. Repeat the experiment three times with every formulation shown in Table 3.
When powder is blended with water or comes into contact with it, an effervescence forms in a matter of seconds. The solution has a pH value that falls between 4 and 5.5. The solution’s acidic flavor (sour-sweet) makes you feel active fresh and reviving.
Drug compatibility
Pharmaceutical incompatibility is the combination of components in which the medical compounds’ physical and chemical characteristics change and, as a result, impair the stability and effectiveness of the medication. This interaction occurs between the medicinal substances and the excipients.
Drug:excipient ratio is 1:1 or 0.5:0.5.
By using infrared (IR) spectrophotometry, the medication and excipient compatibility investigations were conducted.
The purity is also visible in the IR region.
After one month in the stability chamber with the medication and excipient samples, a second IR interpretation of the sample was taken to compare with the first.
Fourier transform infrared spectroscopy
By using Fourier transform infrared spectroscopy (FT-IR) spectroscopy, our investigation was finished (Shimadzu IR Affinity 1 S CE). Drug–excipient interactions were looked at through this study. Samples of the pure medication and excipients were used to scan the spectral spectrum from 4,000 to 400 cm–1 with a resolution of 4 cm–1.
Several noticeable and distinguishable peaks of L-carnitine were visible. Stretching vibrations of the secondary alcohol’s O-H and C-O bonds, respectively, were the cause of the peaks at 3,700 and 3,855 cm–1. Peaks at 2,980, 3,300, and 1,740, respectively, might be attributed to the asymmetric C-H stretching of the CH3 group, the symmetric C-H stretching of the CH2 group, and the C = O stretching.
It was CH3 bending that caused the peaks at 1,414. Because of C-O and C-N stretching, respectively, the maxima at 1058.25 and 1094.62 are reached. There were no interactions between the medication and the excipients in the improved formulation since all of the L-distinctive carnitine’s peaks were present.
Matcha green tea
Catechins are abundant in matcha green tea. It functions naturally as an antioxidant. Additionally, it lowers the chance of developing chronic diseases and aids in the prevention of cell damage.
Additionally, it aids in maintaining healthy liver function and acts as a brain booster by enhancing brain activity. Additionally, it aids in preventing cancer disorders. Breast cancer cell proliferation was inhibited and tumor size was reduced by green tea extract.
Additionally, it benefits cardiac health. Matcha green tea is also used by those with heart function issues to help their hearts operate normally. Additionally, it aids in weight loss and the burning of additional body fat (1).
By combining all the ingredients, creating a powder, and then taking it before exercise, the aforementioned formulation is quite simple to make. Weight loss is aided by it. Additionally, it helps with your everyday exercise and gives your cells energy.
L-carnitine is an essential ingredient in diets for athletes and is important for lipometabolism and the treatment of illnesses that have metabolic problems as well. L-carnitine-rich sports food preparations are frequently used since they greatly improve endurance and supply the muscles with energy.
Such preparations are crucial because they promote muscle activity, which increases endurance and stress tolerance while also postponing exhaustion and speeding up recovery (2).
L-carnitine-containing products can also be used as general dietary supplements and for geriatric purposes, so their use is not just restricted to foods for athletes.
Therefore, the application can essentially be administered parenterally or enterally. When using the preferred enteral, oral.
It is therefore vital to use appropriate forms of administration, preferably in the form of tablets or capsules, but they might also be used in the form of powder or granules.
Regardless of whether the administration method is being used for therapeutic or food purposes, the production in this case is carried out using pharmaceutical technology (3).
The high hygroscopicity of L-carnitine has made the fabrication and processing of such forms of administration up to this point substantially more challenging.
Because they would liquefy quickly even with the natural moisture in the air, tablets containing L-carnitine, for instance, must be made without moisture and packaged hermetically and individually.
Additionally, trimethylamine, which has an unpleasant fishy odor and is frequently found in tiny amounts in L-carnitine, has a repulsive effect on users.
It has been discovered that L-carnitine-L-tartrate is stable in storage and can be handled without the need for extra safeguards when the ambient air moisture is less than or equal to 60% relative humidity (4).
L-carnitine-L-tartrate crystallises into a powder that is easy to process and is especially well suited for processing with machines that operate quickly because it doesn’t tend to bunch up or stay together.
Additionally, it has no smell at all and a refreshing taste because of the bound tartaric acid. slightly acidic flavor.
It is desirable to use L-carnitine-L-tartrate alone or in combination with other active ingredients, such as vitamins, amino acids, trace elements, or mineral compounds, as well as, if desired, the adjuvants typical for the particular form of administration (5).
All types of tablets, including those that need to be chewed or sucked on as well as those that need to be dissolved in a liquid before being taken, are included in the modalities of administration.
The tablet shapes include coated tablets like film tablets or dragees as well as uncoated tablets in one-layer, multi-layer, or enclosed forms. Soft or hard gelatin capsules are additional preferred methods of delivery (6).
Hard-shell capsules made of hard gelatin are the most desired type of these.
L-carnitine-L-tartrate can also be used profitably in powder form, such as granulated powder or effervescent powder, when combined with gas-producing chemicals (7).
Adjuvants include, for example, fillers, binding agents, lubricants, mold release agents, flow-regulating agents, tablet disintegrants, as well as flavoring and coloring compounds.
Persons knowledgeable in the field are familiar with these adjuvants, how to use them, and the technology used to create the aforementioned methods of administration (8).
Results
In terms of appearance, taste, and aroma, the formulations were acceptable. As a result, the L-carnitine in the matcha green tea effervescent powder has the potential to be used as a nutraceutical product, dietary supplement, and anti-diabetic. For patients with type 2 diabetes, such as improving glucose tolerance or lowering fasting blood glucose levels.
Conclusion
According to the study, L-carnitine effervescent tablets were most stable when kept at 4°C, and their most acceptable shelf life was 510 days at that temperature and 425 days at room temperature. Additionally, the F10 formulation of effervescent tablets has an angle of repose that is less than 30, which indicates good flowing properties, and it dissolves quickly in aqueous solution. Granules could therefore be another dosage form for an antidiabetic drug.
Granular powder has a quicker effect than tablet doses since tablets take longer to dissolve. The main disadvantage of tablet dosage forms compared to granule or powder dosage forms is this.
In terms of appearance, taste, and aroma, the formulations were acceptable. Because of this, the L-carnitine effervescent tablet may be utilized as a nutraceutical, dietary supplement, or anti-diabetic. For those with type 2 diabetes, enhancing glucose tolerance or reducing fasting blood sugar levels are two examples.
L-carnitine improved cardiac function in people with heart failure. Carnitine is necessary for a baby’s healthy growth and development.
The new preparations of L-carnitine have less hygroscopicity, longer stability, and a higher storage capacity.
L-carnitine-L-tartrate forms a crystalline powder that is easy to process and is especially suitable for processing with fast-running machines because it does not stick together or become lumpy.
Powder forms of L-carnitine-L-tartrate are favorable, such as granulated powder or effervescent powder with gas-producing ingredients.
The effects of L-carnitine and matcha green tea on metabolism and weight loss are substantially greater.
The granules may be used as a different dose form for pharmaceutical anti-diabetic, nutraceutical, and nutritional supplement products. Granular powder dosage forms work faster than tablets because they dissolve more quickly. Powder dosage forms are also simpler to give and have fewer adverse effects than chemical dosage forms. This is the main disadvantage of tablet dose forms compared to granule or powder dosage forms.
Discussion
Oral drug administration has long been acknowledged as the most common method for the systemic release of medications via various pharmaceutical products of various dosage types. It is feasible to attribute the oral route’s success in part to how easy it is to administer. However, 1–2 Oral continuous medicine administration methods are hampered by short gastric residence times (GRTs). Rapid gastro-intestinal (GI) transit might hinder complete medication release in the absorption zone and reduce the effectiveness of the provided dosage.
Effervescent tablets are described as having the intention of dissolving or dispersing in water prior to distribution. Due to how simple it is to take them, effervescent pills are becoming more and in a number of industries, including supplements and pharmaceuticals. Effervescent pills are made to burst when they come in contact with liquids like juice or water, frequently dissolving into a solution.
An amino acid derivative called L-carnitine is used as a supplement, a vitamin, and in diabetics. It is necessary to maintain the therapeutic dose for 12 h. The main benefits of effervescent tablets include higher patient compliance and good therapeutic concentrations since effervescent dosages release the entire medication in just a few seconds.
Therefore, an L-carnitine formulation with effervescence that releases the medication over the course of 24 h is advantageous owing to its 24–45 h extended half-life.
Effervescent tablets are made with excipients like sodium bicarbonate and carbonate as a base, citric acid, malic acid, fumaric acid, and other sources of acid, as well as sweeteners, flavors, and colors.
As a result, the objective of the current research was to comprehensively examine the impact of formulation variables on the release of L-carnitine effervescent tablets.
Author contributions
AN: theme designing and direction and preparation of the manuscript. SR: visualization and critical review of the manuscript. KS: visualization. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to the whole batch of the Pharmaceutics Department (M. Pharmacy) at Sanjivani College of Education & Research in Kopargaon, India, for their kind support from time to time. We are also thankful to the research guide and mentor for guiding us in our work and to the entire staff of Sanjivani College of Education & Research in Kopargaon, India.
References
1. Lohninger A, Sendic A, Litzlbauer E, Hofbauer R, Staniek H, Blesky D, et al. Endurance exercise training and L-carnitine supplementation stimulates gene expression in the blood and muscle cells in young athletes and middle aged subjects. Monatsh Chem. (2005) 136:1425–42. doi: 10.1007/s00706-005-0335-6
2. Malaguarnera M, Cammalleri L, Gargante M, Vacante M, Colonna V, Motta M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. (2007) 86:1738–44. doi: 10.1093/ajcn/86.5.1738
3. Sawicka A, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek R. l-Carnitine supplementation in older women. A pilot study on aging skeletal muscle mass and function. Nutrients. (2018) 10:255. doi: 10.3390/nu10020255
4. Samulak J, Sawicka A, Hartmane D, Grinberga S, Pugovics O, Lysiak-Szydlowska W, et al. L-Carnitine supplementation increases Trimethylamine-N-oxide but not markers of atherosclerosis in healthy aged women. Ann Nutr Metab. (2019) 74:11–7. doi: 10.1159/000495037
5. Olek R, Samulak J, Sawicka A, Hartmane D, Grinberga S, Pugovics O, et al. Increased Trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women. Oxid Med Cell Longev. (2019) 2019:6247169. doi: 10.1155/2019/6247169
6. Bordoni L, Sawicka A, Szarmach A, Winklewski P, Olek R, Gabbianelli R. A pilot study on the effects of l-Carnitine and Trimethylamine-N-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. Int J Mol Sci. (2020) 21:1047. doi: 10.3390/ijms21031047
7. Grunewald K, Bailey R. Commercially marketed supplements for bodybuilding athletes. Sports Med. (1993) 15:90–103. doi: 10.2165/00007256-199315020-00003
8. Hawley J, Brouns F, Jeukendrup A. Strategies to enhance fat utilisation during exercise. Sports Med. (1998) 25:241–57.
9. Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, et al. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. (2002) 318:51–61.


