Introduction
The primary characteristics of diabetic ketoacidosis are ketoacidosis and hyperglycemia, whereas hyperglycemia in hyperglycemic hyperosmolar non-ketotic syndrome is typically more severe than diabetic ketoacidosis but lacks ketoacidosis (1). The range of 285–295 mOsm/L is considered typical for plasma osmolality (2). The differences between hyperglycemic hyperosmolar non-ketotic syndrome and diabetic ketoacidosis are explored in turn below.
Diabetic ketoacidosis
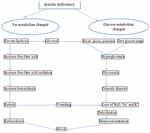
As a result of excessively high blood glucose levels, which might result from inadequate insulin, diabetic ketoacidosis is more common in people with insulin-dependent diabetes mellitus (3, 4). When the body produces high quantities of blood acids called ketones, extremely high blood sugar, and low insulin levels, it can lead to a dangerous complication of diabetes called diabetic ketoacidosis (5). Due to its severe, quick, and 24-h rapid onset, diabetes-related ketoacidosis is regarded as an acute complication (6). Blood sugar levels are greater than or equal to 300 mg/dl in diabetic ketoacidosis (7). Diabetic ketoacidosis only develops when there is insufficient insulin in the body to convert blood sugar into energy. The acids produced by this process—known as ketones— are subsequently used by the liver to digest fat for energy. Diabetic ketoacidosis has a plasma osmolality of less than 320 mOsm/L. In diabetic ketoacidosis, plasma osmolality is frequently increased to more than 290 mOsm/L) (8). The arterial blood’s pH is less than or equal to 7.3 when a person has diabetic ketoacidosis. Although a milder form of diabetic ketoacidosis may present with a bicarbonate level between 15 and 18 mmol/L,a lower PH is frequently associated with a fall in bicarbonate to 15 mmol/L or less (9). The relationship between blood pH and the partial pressure of carbon dioxide in diabetic ketoacidosis is shown in Figure 1 (10). In diabetic ketoacidosis, the blood ketone level was extremely high (11). High blood glucose levels are caused by glucose that cannot enter the cells, which builds up in diabetic ketoacidosis (12). Diabetic ketoacidosis also causes abnormally high urine ketone levels. Osmotic diuresis, which causes a high amount of urine to be produced and causes volume depletion and dehydration, occurs when an excessive amount of glucose reaches the renal tubules in diabetic ketoacidosis (13). Lack of insulin causes too many ketones to accumulate in the blood and eventually “spill over” into the urine (14). The production of ketone bodies in the liver increases when people are fasting or have medical disorders like diabetes mellitus that cause the body to produce more ketone bodies than it can use. When people urinate, the body tries to get rid of them, which causes ketonuria, or excessive ketone levels in urine (15). Diabetic ketoacidosis causes the breath to smell sweet or like acetone (nail polish), and the urine to smell like rotting apples (16). Urine that smells sweet or fruity is an indication of diabetes mellitus or high blood sugar. The sweetness in the urine is caused by sugar and indicates the body is attempting to eliminate surplus sugar in the blood (17). Normally non-existent sugar and ketones can build up in the urine in advanced insulin-dependent diabetes mellitus and produce a pungent odor (18). When the body breaks down fatty acids for energy, ketosis is formed. The liver then excretes ketones as waste products, compromising acetone (19). Because the body is releasing acetone as fat is broken down, the breath may smell nicer (20). Low potassium levels, also known as hypokalemia; cerebral edema, which is an enlargement of the brain; and pulmonary edema, which is an enlargement of the lungs, are complications of diabetic ketoacidosis (21) (Figure 1).
Hyperglycemic hyperosmolar non-ketotic syndrome
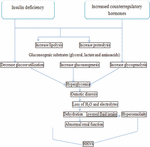
Non-insulin-dependent diabetes mellitus may be more likely to cause hyperglycemic hyperosmolar non-ketotic syndrome (22). People with diabetes mellitus have excessive blood levels of glucose (sugar). Their bodies either do not produce enough insulin or have difficulties using the insulin they do produce, which causes the blood glucose to rise. More frequently, those with non-insulin-dependent diabetes mellitus who do not have their diabetes under control will experience hyperglycemic, hyperosmolar, non-ketotic syndrome (23). The majority of cases of hyperglycemic hyperosmolar non-ketotic syndrome are seen in people with non-insulin-dependent diabetes mellitus who also have another concurrent condition that reduces fluid intake (24). Hyperglycemic hyperosmolar non-ketotic syndrome develops slowly rather than suddenly (25). The blood sugar level in the hyperglycemic hyperosmolar non-ketotic syndrome is greater than or equivalent to 600 mg/dl. When blood glucose levels are extremely high, excess glucose is excreted in the urine (26). In the hyperglycemic hyperosmolar non-ketotic condition, the plasma osmolality exceeds 320 mOsm/L. Higher blood osmolality in hyperglycemic hyperosmolar non-ketotic syndrome is associated with significant impairment of the state of consciousness (27). The PH of arterial blood is greater than or equal to 7.3 in hyperglycemic hyperosmolar non-ketotic syndrome. Acidosis is mostly brought on by dehydration and impaired end-organ perfusion in hyperglycemic hyperosmolar non-ketotic syndrome (28). Hypernatremia is haphazardly associated with a water deficit in people with hyperglycemic hyperosmolar non-ketotic syndrome because of osmotic diuresis-induced hypotonic losses, which cause a loss of water greater than a loss of sodium. Because blood ketones increased when blood glucose levels were high due to a lack of insulin, which is necessary to allow glucose to enter the cells for energy, the hyperglycemic hyperosmolar non-ketotic syndrome showed little or no change in blood ketones (29). Ketosis may not occur in non-insulin-dependent diabetes mellitus patients; ketones may not be produced, or the absence of ketosis may be caused by a relative rather than an absolute lack of insulin, which inhibits the production of ketones. The body attempts to eliminate extra glucose in the urine more often as blood glucose levels rise, which exacerbates dehydration (30). Ketone levels are frequently normal or only slightly elevated because the pancreas produces just enough insulin to maintain fat in fat cells and prevent ketone production (31). In the hyperglycemic, hyperosmolar, non-ketotic condition, the urine ketone level was normal or slightly elevated (32). In the hyperglycemic, hyperosmolar, non-ketotic condition, breath and urine odors are normal (33). The hyperglycemic, hyperosmolar, non-ketotic condition can be complicated by shock, blood clot development, cerebral edema (swelling of the brain), an elevated blood acid level, or lactic acidosis (34) (Figure 2).
Conclusion
When the body produces excessive amounts of blood acids called ketones, extremely high blood sugar, and low insulin levels, it can result in diabetic ketoacidosis, a serious complication of the disease. High blood glucose levels occur as a result of the inability of glucose to enter the cells in diabetic ketoacidosis. In non-insulin-dependent diabetes mellitus, hyperglycemic hyperosmolar non-ketotic syndrome is more common. In contrast to a sudden onset, the hyperglycemic hyperosmolar non-ketotic syndrome develops gradually.
Competing interests
The author has no financial or proprietary interest in any of material discussed in this article.
References
1. Bereda G. Case report: diabetic ketoacidosis during pregnancy due to insulin omission. Open Access Emerg Med. (2022) 14:615618.
2. Rasouli M. Basic concepts and practical equations on osmolality: biochemical approach. Clin Biochem. (2016) 49:936–41.
3. Munoz C, Floreen A, Garey C, Karlya T, Jelley D, Alonso G, et al. Misdiagnosis and diabetic ketoacidosis at diagnosis of type 1 diabetes: patient and caregiver perspectives. Clin Diabetes. (2019) 37:276–81.
4. Balaji R, Duraisamy R, Kumar M. Complications of diabetes mellitus: A review. Drug Invention Today. (2019) 12:1.
5. Dhatariya K, Glaser N, Codner E, Umpierrez G. Diabetic ketoacidosis. Nat Rev Dis Primers. (2020) 6:1–20.
6. Holt R, DeVries J, Hess-Fischl A, Hirsch I, Kirkman M, Klupa T, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. (2021) 44:2589–625.
7. Ayub A, Ijaz S, Qudrat S, Rani T, Raziq A, Ali M, et al. The Impact of COVID 19 Pandemic on Type 1 Diabetes Mellitus: An experience of a Tertiary Care Hospital in a resource limited country. ESPE Abstracts. (2021) 2021:94.
8. Bereda G. Diabetic Ketoacidosis: Precipitating Factors, Pathophysiology, and Management. Biomed J Sci Tech Res. (2022) 44:35843–8.
9. Mahler S, Conrad S, Wang H, Arnold T. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. (2011) 29:670–4.
10. Bereda G. Clinical Management of Gestational Diabetes Mellitus. J Diabetic Nephrop Diabetes Manage. (2022) 1:1–10.
12. Dwivedi M, Pandey A. Diabetes mellitus and its treatment: An overview. J. Adv. Pharmacol. (2020) 1:48–58.
13. Ravindran S, Munusamy S. Renoprotective mechanisms of sodium glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J Cell Physiol. (2022) 237:1182–205.
14. MacFarlane B. Need to know vs good to know: The glucuretics. AJP: The Australian Journal of Pharmacy. (2016) 97:92–5.
15. De Cabo R, Mattson M. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. (2019) 381:2541–51.
16. Warren C. What the dog knows: scent, science, and the amazing ways dogs perceive the world. Simon Schuster. (2015) 2015:10.
17. Karamanou M, Protogerou A, Tsoucalas G, Androutsos G, Poulakou- Rebelakou E. Milestones in the history of diabetes mellitus: The main contributors. World J Diabetes. (2016) 7:1.
18. Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Rad Res. (2013) 47:3–27.
19. van der Kolk J, Gross J, Gerber V, Bruckmaier R. Disturbed bovine mitochondrial lipid metabolism: A review. Vet Quart. (2017) 37:262–73.
20. Mathew T, Pownraj P, Abdulla S, Pullithadathil B. Technologies for clinical diagnosis using expired human breath analysis. Diagnostics. (2015) 5:27–60.
21. Bereda. Risk Factors, Complications and Management of Diabetes Mellitus. Am J Biomed Sci Res. (2022) 16:409–12.
22. Tittel S, Sondern K, Weyer M, Poeplau T, Sauer B, Schebek M, et al. Multicentre analysis of hyperglycaemic hyperosmolar state and diabetic ketoacidosis in type 1 and type 2 diabetes. Actadiabetologica. (2020) 57:1245–53.
23. Fayfman M, Pasquel F, Umpierrez G. Management of hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin. (2017) 101:587–606.
24. Garg S, Peters A, Buse J, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Therapeut. (2018) 20:571–5.
25. Bereda G, Bereda G. The Incidence and Predictors of Poor Glycemic Control among Adults with Type 2 Diabetes Mellitus in Ambulatory Clinic of Mettu Karl Referral Hospital, South Western, Ethiopia: A Prospective Cross Sectional Study. Int Arch Endocrinol Clin Res. (2021) 7:024.
26. Hensen J. Diabetic Coma: Current Therapy of Diabetic Ketoacidosis and Non-Ketoacidotic Hyperosmolar Coma In Type 2 Diabetes. Boca Raton, FL: CRC Press (2016).
28. Whitmore S, Gunnerson K. Acid-Base and Electrolyte Disorders in Emergency Critical Care in Emergency Department Critical Care. Cham: Springer (2020).
29. Dhatariya K, Vellanki P. Treatment of diabetic ketoacidosis (DKA)/hyperglycemic hyperosmolar state (HHS): novel advances in the management of hyperglycemic crises (UK versus USA). Curr Diabetes Rep. (2017) 17:1–7.
30. Bereda G. Hyperosmolar Hyperglycemic State: Background, Precipitating Factors, Pathophysiology and Management. In J Dia It Compl. (2022) 1:1–6.
31. Kolb H, Kempf K, Rohling M, Lenzen-Schulte M, Schloot N, Martin S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. (2021) 19:313. doi: 10.1186/s12916-021-02185-0
32. Pasquel F, Umpierrez G. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. (2014) 37:3124–31.
33. Bereda G. Complication of Diabetes Mellitus: Microvascular and Macrovascular Complications. Int J Diabetes. (2022) 3:123–8.
34. Wolfsdorf J, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. (2018) 19:155–77.