Introduction
As global food consumption continues to rise in the coming years. Global food demand will increase approximately 60% by 2050 compared to 2005, according to the United Nations’ Food and Agriculture Organization (1). Crop production has to be increased in agriculture to fulfill global food demand. One of the most efficient ways to spur plant growth is fertilizer, which can play a significant role in increasing crop productivity (2). Environmental pollution is caused by fertilizer use, which results in health problems. Several factors contribute to environmental pollution, including the high solubility of nutrients and their leaching followed by soil mobility (3).
Plants can only use a small number of chemical fertilizers because they are highly soluble in water. Several processes affect the rest of them when they are applied to a field, such as adsorption, degradation, runoff, and leaching. In other words, soluble chemical fertilizers are quite ineffective. Because fertilizers are derived from inorganic anions such as nitrate and phosphate, excessive application of fertilizers can cause contamination of surface water and soil (4).
There have been several types of slow-release fertilizers made from different materials, including various minerals (5–8). Slow-release mineral fertilizers have gained a lot of attention for their environment-friendly properties and ability to maintain soil fertility. Minerals are usually charged to protect nutrients from rapid degradation as well as having a crystal structure (8–13).
The mineral feldspar is the most common one found in rocks. A group of minerals that are known collectively as feldspar is a mineral with the chemical formula x Al (Al, Si)3O8, where x might be calcium (Ca), sodium (Na), or potassium (K). Because of their high alumina and alkali contents, feldspars are predominantly utilized in industrial applications (14). Feldspar is used to describe a wide range of materials. We use feldspar in a substantial number of everyday products, including drinking glasses, windshields, fiberglass for insulation, bathroom floor tiles, shower basins, and even the dishes on our tables. Feldspar is ubiquitous. An infinite number of tetrahedral SiO2 and AlO4 networks comprise the crystal structure of feldspars (14).
According to Zhang et al. (15), the minerals that contain at least some albite or anorthite are called plagioclase feldspars. In contrast, those that include alkali feldspars are known as orthoclase feldspars (16). Due to feldspars’ industrial application, the latter category is especially interesting. Depending on the type of alkali they contain, feldspars can also be divided into sodium, potassium, and mixed types (15). Thermal gravimetric analysis (TGA), Fourier transforms Infrared (FT-IR), and x-ray diffraction (XRD) were used to explain the unique properties of Feldspar (17).
There is a wide range of applications for feldspars, from fluxing agents for ceramics to fillers for paint, rubber, and adhesive industry purposes (14, 18). Therefore, this research aims to optimize control/slow-release fertilizers (C/SRFs) by combining feldspar minerals with KH2PO4 fertilizers using a dry mechanochemical approach. We aim to highlight the significance of adjusting the milling time and rotation speed in the synthesis of feldspar slow-release fertilizers by studying their mechanochemical properties.
Experimental sections
Mechanochemical processes and materials
High-purity feldspar from the Yutum Granite Suite (KAlSi3O8) (part of the Aqaba complex) (14, 19) and Pan- reac, PRS undergoes mechanical reactions with potassium dihydrogen phosphate (KH2PO4). Ball mill, planetary (Pulverisette-7, Fritsch, Germany), was used for milling under a choice of time and rotation speed, using 6.0°g (3:1) feldspar/fertilizer and 7 steel balls of 15°mm diameter. In each experiment, alternating 10°min of milling with 5°min of rest was done to prevent excessive heat from accumulating during milling. Two discussed parameters were studied in Table 1.
Characterization
X-ray diffraction measurements were performed on the Feldspar-KH2PO4 samples before and after milling. Radiation was Cu K has a range of 2° between 5 and 90°. The produced samples were examined using infrared spectrometers as the NEXUS and the EPS-870. At a resolution of 2°cm–1, the sensors were scanned from 4,000 to 500°cm–1 an advanced sensor (not KBr). Using a NETZSCH STAT-409 PC thermal gravimetric analyzer in an N2 atmosphere, the analysis was performed in 10°C/min increments from room temperature to 1,000°C.
A 100-ml glass beaker was used to conduct the leaching tests with KH2PO4 ground in distilled water. The parameters were the following: 1.0°g of ground sample, 20°ml of distilled water, and 24°h of incubation. Vacuum filtration with 0.45°m pore size filter paper was used after leaching to separate solids from liquids. The parameters of the liquid ion chromatography were determined by the concentration of the K+, NH4+, and PO34– ions (nutrients) in the filtrate along with determining the total nitrogen released from urea by the Kjeldahl Nitrogen Determination instrument (IC, Thermo Scientific, column series Dionex, CS-5000 + DP, Germany).
Results and discussion
Hae-Jin Jung, Md Abdul Malek, JiYeon Ryu, BoWha Kim, Young-Chul Song, HyeKyeong Kim, and Chul-Un Ro.
There are no studies available providing data on slow- release fertilizer systems synthesized from feldspar and fertilizers. More research is needed on the effectiveness of different parameters in fertilization. During amorphous material developed and reacted with salts during the production of feldspar, changing the solubility profile of compounds and generating new interactions. It has been established that feldspar has a neutral, layered alkaline structure that does not interact with other substances, including the study’s target phosphate salts.
Feldspar-KH2PO4 System
We investigated the influence of the milling speed and the milling time in the high-energy ball mill. To achieve the most suitable conditions for utilizing the slow-release properties of nutrients, a series of experiments were conducted with feldspar and selected fertilizers.
Mechanochemical synthesis of Feldspar-KH2PO4 as SRF - effect of milling speed
In this study, the phosphate salts of interest were studied as amorphous feldspars produced by the formation of polymorphous aggregates. The emergence of amorphous materials changed the solubility profiles of the salts and enhanced interactions between the products that were obtained. As shown in Figure 1, amorphization of the Feldspar-KH2PO4 (3:1 weight ratio) sample mixture is influenced by mill rotational speed. The mill rotational speed ranged between 100 and 600°rpm for all experimental runs, with a fixed milling time of 120°min. When mill rotation speeds between 100 and 400°rpm were lowered, characteristic patterns related to KH2PO4 were still visible in the milled products. The higher mill speeds yielded a complete amorphous reduction of the samples, which suggests the amorphous structure of feldspar incorporates KH2PO4.

Figure 1. An XRD pattern of the Feldspar-KH2PO4 sample mixture was milled at varying mill rotational speeds for 120°min.
Each of the experimental runs was run for 120°min with mill rotation speeds ranging from 100 to 600°rpm for the FT-IR spectra to be collected. The characteristic spectrums of KH2PO4 at 1,281°cm–1 remained in the milled products at lower mill rotational speeds, such as 100−400°rpm, as shown in Figure 2. As a result, the clear band at 1,281°cm–1 on the KH2PO4 spectrum was eliminated from sample mixtures that were milled at speeds of 400°rpm, which suggests successful intercalation of KH2PO4 in feldspar, distinctive due to the overlap of Al-OH, Si- O, and P-O vibrations, wide bands were seen in the area approximately 1,000°cm–1 for all samples (20–22).

Figure 2. Fourier transform infrared spectroscopy (FTIR) spectra of samples Feldspar-KH2PO4 milled at varied rotating speeds for 2°h.
Moreover, the shoulder band of feldspar at (1,100°cm–1) vanished when milling over 400°rpm, which indicates a better mechanochemical reaction, occurs at high milling speeds than at low speeds. The thermal gravimetric analysis (TGA) patterns of Feldspar-KH2PO4 samples that were milled for 2°h at different milling speeds were shown in Figure 3.
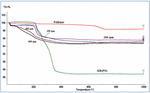
Figure 3. The TGA patterns of Feldspar-KH2PO4 sample mixtures were milled for 2°h at various mill speeds.
Figure 3 shows that feldspar did not present significant mass loss during TGA analysis, with a mass loss of 0.5%. This small loss shall be related to the evaporation of impurities like carbonates and the free water elimination of feldspar, which is a good match for the predicted value of 0.3% (10), demonstrating the high purity of the feldspar that was employed. The phosphate salts generally disintegrate in three steps, starting endothermic peaks at 230, 270, and 360°C for potassium dihydrogen phosphate (Figure 3). For samples milled at 100 and 200°rpm, clear mass loss obtained at 230°C illustrated that these low speeds of rotation were not enough to incorporate KH2PO4 into feldspar, Nevertheless, no mass loss characteristics of the raw materials were observed in the milled samples of potassium dihydrogen phosphate milled at 400 and 600°rpm, which indicates that they are destroyed during grinding; this is in agreement with the observations of other instrumental techniques showing that amorphous phases were formed by combining the reagents.
Figure 4 depicts the 24-h release curve of K+ and PO34– nutrients from Feldspar-KH2PO4 sample mixes milled at varied rotating rates can be determined. As shown by the results from samples prepared at 100 and 200°rpm, the release of both K+ and PO43– reached around 1,003 and 983°ppm, respectively. These results indicate a greater mill rotational speed is required for complete amorphization of the starting materials to incorporate KH2PO4 into the amorphous feldspar structure. As the mill speed was increased from 400 to 600°rpm, the release of kaolin and phosphate nutrients into the solution decreased markedly. This was achieved by reaching 974°ppm at 400°rpm and sharply decreasing to 484°ppm at the sample. This suggests 600°rpm, suggesting the addition of KH2PO4 to feldspar in amorphous form. Specifically, metals such as Al and Si, especially those that are present in Al-Si-O networks, retard the diffusion movement of these elements (23). In water, both K+ and PO34– dissolve as a result of KH2PO4, and these chains are attached to Al-Si-O molecules.
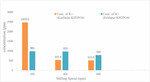
Figure 4. The profile of nutrient release of feldspar-KH2PO4 samples, determined after milling at a variety of mill speeds for 2°h and dispersing them in water for 24°h.
A correlative study with Kaolinite-KH2PO4 (5) is investigated to make sense and clarify the effect of milling speed at a fixed wt ratio and milling time to the release of K+ from different complexes when dispersed into distilled water for 24°h; the results are displayed together in Table 2.

Table 2. Collected results of K+ nutrients in (ppm) released from KH2PO4 incorporated with kaolinite/feldspar, milling for 120°min at a different milling speed.
Figure 5 clarifies the correlation process between Kaolinite-KH2PO4 (5) and Feldspar-KH2PO4 complexes results in Table 2, examining the effectiveness of these complexes as SRF by milling at 600°rpm for various time intervals and comparing the nitrogen release results to determine which one is the most suitable as SRF.

Figure 5. Correlation process between conc. K+ released from Kaolinite-KH2PO4 and Feldspar-KH2PO4 complexes with different milling speed.
Figure 5 and Table 2 demonstrate that it is possible to reduce disintegration of K + nutrients by increasing milling speed, which is in agreement with IR, TGA, and XRD results (5); however, results refer that Kaolinite-KH2PO4 is more suitable than feldspar as a nutrient carrier and best for use and application as SRF.
Feldspar-KH2 PO4 as SRF: Effect of milling time
Amorphization of the Feldspar-KH2PO4 sample mixture was studied using the XRD patterns shown in Figure 6. Throughout all experimental runs, milling speed was fixed at 600°rpm, and milling intervals ranged from 60 to 180°min. As can be seen in Figure 6, most of the characteristics associated with KH2PO4 had been removed from milled products at a lower milling time of about 60°min. Furthermore, using a 120°min milling time, KH2PO4 was reduced to an amorphous state and the featured peaks disappeared, supporting the idea that KH2PO4 was incorporated into the amorphous structures of the feldspar.

Figure 6. The XRD patterns of Feldspar-KH2PO4 samples were milled for different periods at a speed of 600°rpm.
As can be seen in Figure 7, the thermal dehydroxylation of the feldspar caused a mass loss of 0.3%. In the milled samples, potassium dihydrogen phosphate, the mass loss characteristics were not observed, which could indicate their destruction in the process of grinding. This was confirmed by other instrumental methods that showed that amorphous phases originated from the reagent mixture. Additionally, Figure 7 shows that 3°h of milling resulted in just one mass loss event; however, samples milled for 60 and 120°min showed two additional mass losses, suggesting that milling duration affects mechanochemical synthesis.

Figure 7. Patterns of TGA measurements on Feldspar-KH2PO4 samples milled at 600°rpm for different periods.
Figure 8 illustrates how nutrients are released from samples milled to 600°rpm as a function of milling time after 24°h of leaching in distilled water. Both K+ and PO43– were detected at over 927°ppm in sample mixtures that were prepared after 60°min of milling time. This indicates sufficient milling time was required to cause complete amorphization of the starting materials to allow KH2PO4 to be incorporated into the amorphous feldspar structure.

Figure 8. A nutrient release profile for Feldspar-KH2PO4 sample mixtures milled at 600°rpm at different milling times and dispersed in water at different times.
During milling for 180°min, both K+ and PO43– nutrients were released substantially less, reaching around 708 and 653.8°ppm and reaching more than 780°ppm for samples obtained from milling at 120°min. This indicates that KH2PO4 had sufficient time at 120°min to incorporate into the structure of feldspar. Milling for long periods may cause the compound to escape slightly from feldspar.
Conclusion
A novel Feldspar-KH2PO4 mixture loaded with KH2PO4 demonstrated remarkable mobility in solution because of the nutrient release characteristics of KH2PO4, which confirmed that, in addition to being simple and economical, the mechanochemical route also proved to be effective for synthesizing Feldspar-KH2PO4 complex samples that act as intelligent fertilizers. By optimizing the ratio of feldspar to fertilizer (3:1 wt ratio) and milling speed at 600°rpm for 2°h, this study illustrated the ability to open a novel approach to the preparation of SRF and higher possibilities for the preparation of low-cost precursor biomass material. The nutrient results indicated that KH2PO4 had a sufficient time and milling speed to incorporate KH2PO4 into the structure of feldspar.
Acknowledgments
The University of Jordan and Tafila Technical University are greatly acknowledged for their support of this research.
References
1. Saji S, Umemoto T, Kida H, Sakata K. Antitumor effect in peritoneal carcinomatosis of intraperitoneal administration of a streptococcal preparation, OK-432: I. Experimental study in the rat. J Surg Oncol. (1985) 30:46–51. doi: 10.1002/jso2930300113
2. Alrbaihat MR, Al-rawajfeh AE, Alshamaileh E. A mechanochemical preparation, properties and kinetic study of kaolin - N, P fertilizers for agricultural applications. J Mech Behav Mater. (2021) 30:265–71. doi: 10.1515/jmbm-2021-0028
3. Lim SF, Matu SU. Utilization of agro-wastes to produce biofertilizer. Int J Energ Environ Eng. (2015) 6:31–5. doi: 10.1007/s40095-014-0147-8
4. Olarewaju OE, Adetunji MT, Adeofun CO, Adekunle IM. Nitrate and phosphorus loss from agricultural land: implications for nonpoint pollution. Nutr Cycl Agroecosyst. (2009) 85:79–85. doi: 10.1007/s10705-009-9249-8
5. Al-rawajfeh AE, Alrbaihat MR, Ehab M. Effects of milling time and speed on nutrient. Jordan J Chem. (2020) 15:51–9. doi: 10.47014/15.2.1
6. Boldyrev VV, Tkacova K. Mechanochemistry of solids: past, present, and prospects. J Mater Synth Process. (2000) 8:121–32.
7. Camargo JA, Alonso A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ Int. (2006) 32:831–49. doi: 10.1016/j.envint.2006.05.002
8. Zhang Y, Zou H, Ling Y, Dang X, Yu N, Zhang Y, et al. Solubility characteristics and slow-release mechanism of nitrogen from organic-inorganic compound coated urea. Int J Photoenergy. (2015) 2015:705471. doi: 10.1155/2015/705471
9. Adhikari T. Nanotechnology in environmental soil science. In: Rakshit A, Singh S, Abhilash P, Biswas A editors. Soil Science: Fundamentals to Recent Advances, December 2015. Singapore: Springer (2021). p. 297–309. doi: 10.1007/978-981-16-0917-6_14
10. AlShamaileh E, Al-Rawajfeh AE, Alrbaihat M. Mechanochemical synthesis of slow-release fertilizers: a review. Open Agric J. (2018) 12:11–9. doi: 10.2174/1874331501812010011
11. Periasamy A, Muruganand S, Palaniswamy M. Vibrational studies of Na2so4, K2so 4, NaHSO4and KHSO4 crystals. Rasayan J Chem. (2009) 2:981–9.
12. Said A, Zhang Q, Qu J, Liu Y, Lei Z, Hu H, et al. Mechanochemical activation of phlogopite to directly produce slow- release potassium fertilizer. Appl Clay Sci. (2018) 165:77–81. doi: 10.1016/j.clay.2018.08.006
13. Udvardi B, Al-rbaihat MR, Al Shamaileh EM, Saqarat B. An FTIR spectroscopic study of a novel kaolinite-NPK mixture an FTIR spectroscopic study of a novel kaolinite-NPK mixture. J Eng Sci Comput. (2019) 1:81–93.
14. Aljaradin M, Amareh M. Characterization of the jordanian feldspar raw materials for application in the ceramic and glass industries. Int J Mining Eng Miner Process. (2015) 3:28–31. doi: 10.5923/j.mining.20140302.02
15. Zhang Y, Qu C, Wu J, Lu M, Rao P, Liu X. Synthesis of leucite from potash feldspar. J Wuhan Univ Technol Mater Sci Edit. (2008) 23:452–5. doi: 10.1007/s11595-007-4452-4
16. Qian Z, Hu G, Zhang S, Yang M. Preparation and characterization of montmorillonite-silica nanocomposites: a sol-gel approach to modifying clay surfaces. Phys B Condens Matter. (2008) 403:3231–8. doi: 10.1016/j.physb.2008.04.008
17. Song YC, Ryu J, Malek MA, Jung HJ, Ro CU. Chemical speciation of individual airborne particles by the combined use of quantitative energy-dispersive electron probe X-ray microanalysis and attenuated total reflection Fourier transform-infrared imaging techniques. Analyt Chem. (2010) 82:7987–98. doi: 10.1021/ac1014113
18. Potter MJ, Luty B, Zhou HX, McCammon JA. Time dependent rate coefficients from brownian dynamics simulations. J Phys Chem. (1996) 100:5149–54. doi: 10.1021/jp953229n
19. Al-Harahsheh M, Shawabkeh R, Al-Harahsheh A, Tarawneh K, Batiha MM. Surface modification and characterization of Jordanian kaolinite: application for lead removal from aqueous solutions. Appl Surf Sci. (2009) 255:8098–103. doi: 10.1016/j.apsusc.2009.05.024
20. Azeem B, Kushaari K, Man ZB, Basit A, Thanh TH. Review on materials & methods to produce controlled release coated urea fertilizer. J Control Release. (2014) 181:11–21. doi: 10.1016/j.jconrel.2014.02.020
21. Frost RL, Mako E, Kristof J, Horvath E, Kloprogge JT. Modification of kaolinite surfaces by mechanochemical treatment. Langmuir. (2001) 17:4731–8. doi: 10.1021/la001453k
22. Rudmin M, Abdullayev E, Ruban A, Buyakov A, Soktoev B. Mechanochemical preparation of slow release fertilizer based on glauconite-urea complexes. Minerals. (2019) 9:1–10. doi: 10.3390/min9090507
