1. Introduction
Currently, all authors of articles adhere to the hypothesis Professor Carl von Rokitansky (1842), which explains the presence of clinical and radiological symptoms of obstruction in the 3rd part of the duodenum, by its compression in the aortomesenteric angle (AMA). Therefore, this syndrome has received the name “Superior mesenteric artery syndrome” (SMAS). There are many contradictory statements in this hypothesis, which will be discussed below. To understand the pathological physiology of SMAS, it is necessary to focus on the normal physiology of the duodenum.
2. Duodenal motility
In the duodenum, between the pyloric sphincter (PS) of the stomach and the duodenojejunal junction, four sphincters function, which protects the small intestine from the aggressive effects of hydrochloric acid. If the bolus would pass through the duodenum as quickly as through the esophagus, then an extremely low pH bolus would cause irritation of the jejunum or, at best, a dumping syndrome (1).
2.1. On the physiology of the sphincters of Ochsner and Kapandji
Ochsner described in detail the functional sphincter in the 3rd part of the duodenum in 1906. During gallbladder and stomach operations he found that “in many cases, the duodenum is distended with gas to a point just below the entrance of the common duct, while below this it is contracted, and upon raising the transverse colon and finding the origin of the jejunum, this portion of the intestine will also be found in a contracted condition. In all these specimens, there is also a marked thickening of the intestinal wall at a point 2−4 cm below the entrance of the common duct, and a careful study of this thickening demonstrated the presence of a marked increase in the circular muscle fibers. These facts pointed toward the presence of a sphincter at this point whose physiological function would consist in providing for a means of retaining the chyme in the upper portion of the duodenum sufficiently long to provide for thorough mixing with bile and pancreatic fluid.” Ochsner believed that “under certain forms of irritation or inflammation of the gallbladder or ducts, this duodenal sphincter had taken up an action” (2). The 12 out of 14 patients described by Ochsner were diagnosed with gastric ulcer (1), duodenal ulcer (2), cholecystitis (7), chronic appendicitis (5), and pancreatitis (7). All patients had a dilated duodenum, and 6 of them had a gastric dilatation (2). Kapanji described the sphincter in the 2nd part of the duodenum and explain its functional significance (3).
Kapanji’s sphincter and Ochsner’s sphincter are widely known in the scientific literature in French and Russian (4). Oddi’s sphincter is the only sphincter of the duodenum that is described in the English-language literature. The pathology of this sphincter is closely related to the pathology of the bile and pancreatic ducts.
2.2. X-ray studies of the duodenal sphincters
The motility of the Ochsner’s sphincter is a periodic change of contraction and relaxation, depending on the pH of the bolus. Normally, radiologists cannot see a contraction of the Ochsner sphincter because they use an acid-free contrast agent. I have done research adding 3g Vitamin C to 200 ml of barium. The acid caused Ochsner’s sphincter to contract and this made possible to determine and measure of its length. It turned out that in length (3.20 ± 0.15 cm) and location it corresponds to the length (3.30 ± 0.15 cm) (P > 0.2) and location of the narrowing in the duodenum at the SMAS (4, 5).
2.3. The normal duodenal motility
The post bulbar sphincter (PBS), together with the pyloric sphincter (PS), provides evacuation of the chyme from the stomach as portions of a certain volume (Figure 1A). When the acid bolus reaches the Ochsner’s sphincter, which is in the 3rd part of the duodenum, it causes of its contraction, which prevents entering aggressive chyme to the jejunum (Figure 1B). As a result of the Ochsner’s sphincter contraction, the bolus is thrown cranially, but Kapanji’s sphincter contraction pushes it towards the Ochsner’s sphincter. This pendulum movement of the bolus between the Ochsner and Kapanji sphincters occurs several times. During this time, the chyme mixes with bile and pancreatic juice, which raise the pH (Figure 1C) of the chyme. When the pH reaches a level that is safe for the jejunum, Ochsner’s sphincter opens, and bolus passes into the jejunum (4–6). The portioned flow of bile and pancreatic juice into the intestine between the Ochsner and Kapanji sphincters is provided by the sphincter of Oddi. In addition, it prevents the penetration of duodenal contents into the bile ducts (Figure 1).

Figure 1. X-ray demonstration of the duodenal sphincters. (A) During antral contraction, the duodenal bulb fills to the limit, after which the pyloric sphincter (PS) contracts, stopping the flow of barium from the stomach into the bulb. Then, during the peristaltic contraction of the bulb between the PS and PBS, the pressure rises, which causes the PBS to relax, and the bolus penetrates the 2nd part of the duodenum. (B) In an elderly patient with duodenal dyskinesia the white arrow shows PBS location. An expansion of the duodenum is determined between the Kapanji’s sphincter (pink arrow) and the Ochsner’s sphincter (blue arrow). (C) The duodenum was emptied, but the barium remained in deep folds because the barium-filled bowel was very wide. Two zones of contraction with longitudinal folds are visible: (a) the Kapanji’s sphincter, and (b) the Ochsner’s sphincter. The juxtapapillary diverticulum (d) is located between them. These diverticula result from the extrusion of the mucosa between the muscular fibers. Thus, this diverticulum is evidence of high pressure that occurs between contracted sphincters Kapanji and Ochsner.
3. Analysis of the vascular hypothesis of the pathogenesis of SMAS
3.1. Analysis of radiological studies
Ever since Professor Carl von Rokitansky (1842) advanced the vascular hypothesis, it is believed that obstruction of the 3rd part of the duodenum is caused by intestinal compression in the aortomesenteric angle (AMA). The abdominal aorta is located near the middle of the vertebra (Figure 2B). Its width is approximately 2 cm, and the width of the superior mesenteric artery (SMA) is 0.5 cm. The length of the constriction that these vessels can create cannot exceed 1 cm. I measured the distance from proximal boundaries the sharp contraction in the 3rd part of the duodenum to the location of the SMA, i.e., up to the middle of the 3rd lumbar vertebra (L-3) on 35 radiographs, CT, and Magnetic Resonance Imaging (MRI), published in PubMed and PMC. On radiographs, all the values are greater than the real ones. We calculated the true value by multiplying the value measured on the radiograph by the projection increase factor. It is equal to the ratio of the true height of the first lumbar vertebra (2.2 cm in adults) to the value of its image on the radiograph. When analyzing CT and MRI, the coefficient is equal to the ratio of the true diameter of the abdominal aorta (2 cm) to the value of its image on the scan. In 29 (83%) cases on X-ray examination or on CT and MRI, the length of the narrowed segment of the duodenum ranged from 2.5 to 4.6 cm (3.30 ± 0.15 cm) and always started a few centimeters to the right of L-3 (Figures 2A–D) (4–7).

Figure 2. X-ray examinations of patients with a “diagnosis” of SMAS. (A) A patient after laparotomy. The beginning of the narrowed segment of the duodenum (blue line) is located 3.2 cm from the midline L-3. His CT (B) the narrowed segment is located far from the AMA (red line). (C) In a patient with a bulb ulcer (black arrow), the narrowed segment of the duodenum begins to the right of L-3 (white arrow) and ends to the left of L-3. Its length is 4.6 cm. (D) The distance from the duodenum to the jejunum is 6.5 cm. (E) In this patient the narrowed segment of the duodenum starts to the right of L-4 and ends to the left of the L-3 midline. Its length is 4.9 cm.
Only in 6 (17%) of 35 cases where the length of the narrowed duodenal segment could be measured, it looked short, since the place of obstruction was near the midline of the vertebra and its length was within 1 cm. However, as seen in (Figure 2E), the narrowed segment of the duodenum can be located to the left of the midline of the vertebra, which is confirmed by Ochsner’s observations during surgery. Secondly, the X-ray can show the moment opening of the Ochsner’s sphincter, which happens when there is high pressure above it (Figures 3A, B).

Figure 3. (A) MRI of a patient with SMAS and (B) schem to it. The second part of the duodenum is expanded to 4 cm. Deep fixid folds in the third part of the duodenum indicate rigid duodenitis. This picture, resembling a hedgehog, was the same in three patients. The moment of opening of Ochsner’s sphincter was probably recorded at high pressure in the expanded duodenum above it.
Carl von Rokitansky proposed a vascular hypothesis for the pathogenesis of the so-called SMAS almost 200 years ago, when there were no radiological research methods that we use now. Mathematical analysis of radiological studies showed that the narrowed segment of the duodenum in location and length (3.30 ± 0.15 cm) fully corresponds to the location and length (3.20 ± 0.15 cm) of the Ochsner sphincter (P > 0.2).
3.2. Analysis of the clinical picture
It is argued that the cause of the compression of the duodenum in the AMA is rapid weight loss and the disappearance of adipose tissue, which normally supposedly push the SMA away from the aorta (8). This statement is hypothetical in nature, since, firstly, no evidence is provided to support this thesis. On the contrary, analysis of the literature does not support this claim. For example:
In a significant percentage of patients with SMAS, the mean body mass index (BMI) was within normal limits. In a series of 14 children, it was 21.3 kg/m2 (range 13.3−30.4 kg/m2). The mean weight loss before diagnosis was 3.8 kg (range 0−20 kg). No weight loss was found in 50% of the patients (8).
Among patients with acute development of the disease, i.e., within 1−53 (8.2 ± 1.9) days, conservative treatment was successful after 2−59 (13.4 ± 2.9) days in 88.9% of patients. First, it is obvious that during this time the volume of adipose tissue in the AMA could not change significantly. Second, repeated CT after resolution of clinical symptoms did not reveal any changes in AMA (9).
If soft adipose tissue appears between the aorta and the SMA, then how does it push forward the tense vessel and does not push back and squeeze more compliant duodenum?
3.3. Analysis of the significance of the aorto-mesenteric angle
All authors, referring to the article by Neri et al. (10), believe that the AMA value less than 25 is an important diagnostic feature of SMAS. However, the study, to which everyone refers, was carried out with numerous violations that do not allow us to consider its results reliable, that is, scientific. Studies with the AMA <25 were initially selected. No justification for the choice of this figure is provided. There are no characteristics of 50 healthy subjects (control group). The authors did not measure AMA for persons with different BMIs. Therefore, they had no reason to report normal boundaries at all. ≪The US detected reduced angles <25 in 29 of the 950 subjects (3.05%). X-ray with barium (Figure 4A) revealed compression on the third segment of the duodenum in 28 of 29 patients during the symptomatic period and in nine of 29 during the symptom-free interval≫ (10).

Figure 4. (A) Radiograph from the article Neri et al. (10). The stomach and duodenum are not dilated. The contracted segment of the duodenum begins to the right of the spine. (B, C). Radiographs from the textbook. Signed: “A frontal projection shows apparent obstruction of the third portion of the duodenum (arrow) suggesting the SMAS. (C) A right anterior oblique view obtained slightly later shows the duodenal sweep to be entirely normal” (11).
On the radiograph (Figures 4B, C), which Neri et al. (10) presented as the most demonstrative of 29, there are no signs of obstruction because neither the stomach nor the duodenum is dilated. Secondly, the narrowing that begins to the right of the vertebra has nothing to do with AMA. The contraction of the duodenum corresponds to the normal function of the Ochsner sphincter. This is confirmed by a similar observation by Eisenberg (11). Thus, this radiograph does not correspond to the concept of SMAS, since it shows no signs of impaired evacuation through the duodenum. It is a falsification of the facts for confirming the invented limit of the AMA norm - 25. Neri et al. state: - “The aortomesenteric angle is normally 25–60 [(2, 3, 6, 7, 10–12)] and the mean aortomesenteric distance of 10–28 mm [(1–3, 6, 7, 10–12)]. Subjects presenting an angle <25 and an aortomesenteric distance <8−10 mm may be affected by SMA syndrome” (10). However, in none of the cited references, there were studies on the magnitude of ARA in healthy individuals. Moreover, most of the links are descriptions of isolated cases. Thus, the conclusion of Neri et al. (10) that “Subjects presenting an angle <25 and an aortomesenteric distance < 8−10 mm may be affected by SMA syndrome” is not justified, since most individuals with such indicators do not have SMAS.
Bhagirath Desai et al. (12) did a prospective study of 100 patients who had undergone CT scans for various other complaints. A strong positive correlation was found between BMI and the angle between the aorta and SMA. With BMI increase, the angle also increases. In 25% of patients, these rates were less than the norm. In third world countries, there are hundreds of millions of people with low BMI which does not increase the SMAS frequency. Thus, the AMA value depends only on the amount of fat in the body.
Based on the above, we can conclude that: (1) No one has researched the AMA value in healthy people of different ages and weights. (2) The value of AMA is proportional to BMI. (3) The statement that an AMA <25 should be suspected of SMAS is the result of nonconscientious research.
3.4. How does the AMA change?
This question is very important, since another, even more improbable hypothesis about the role of adipose tissue in AMA has been proposed to support the vascular hypothesis. The diagram in Figure 5 in parts A and B shows the principle of this hypothesis. SMAS supposedly arises from the loss of adipose tissue in the AMA, which leads to compression of the duodenum, which passes through this angle. From this follows the principle of SMAS treatment, i.e., hyperalimentation, to increase the volume of adipose tissue and push the SMA anteriorly and thus make room for the duodenum. This hypothesis is rejected by the following evidence. (1) An increase in soft adipose tissue in the AMA cannot push back a tense vessel. (2) An additional volume of adipose tissue competes with the duodenum for space in the AMA, i.e., it can increase its compression. (3) Hyperalimentation can lead to an increase in adipose tissue only with a simultaneous increase in fat throughout the body. This means that for enough fat to appear in the AMA, the patient must gain tens of kilograms. This cannot be done not only in a few days, but also in many months, especially since we are talking about a violation of the patency of the duodenum. Figure 5 provides further evidence that “when BMI of a patient decreases, there is a corresponding reduction in SMA angle and distance between aorta and SMA” (12, 13).
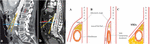
Figure 5. Central sagittal scans (CT). The true height of the 1st lumbar vertebra (red line) is 2.2 cm. (A) In a patient with SMAS, the greatest distance from the anterior abdominal wall to the spine is 8 cm (blue). (B) A patient with SMA thrombosis (red arrow) has a large volume of adipose tissue pushed the anterior wall and colon forward to 13.2 cm (8 cm- blue + 5.2 cm- white) from the spine. The SMA that supplies blood to the colon together with the colon and mesentery is pushed forward, which leads to an increase in AMA (yellow angle). (A, B) Scheme of the generally accepted hypothesis of the pathogenesis of SMAS, which contradicts physical laws. (C) Scheme of the increase in AMA because of pushing the SMA along with the intestine and its mesentery.
4. Clinical analysis of 227 cases of SMAS
I have analyzed 79 articles from PubMed and PMC describing 227 cases of SMAS from 1990 to 2015. Based on clinical data, all patients were divided into 2 groups. The 1st group consisted of 101 patients aged 3−81 (25.8 ± 3.4) years. The obstruction of the duodenum appeared in them 1–53 (8.2 ± 1.9) days after severe stressful events: complicated surgeries, burns, trauma, chemotherapy, etc. In 126 patients of the second group aged 17–86 (36.7 ± 2.2) years, including 8 patients with anorexia nervosa, the duodenal obstruction occurred after 3−72 (17.2 ± 3.2) months of the chronic diseases. Almost all these patients had acid-related upper gastrointestinal tract diseases. It became apparent that two different processes can lead to the appearance of duodenal obstruction (Table 1).

Table 1. Distribution of the patients with superior mesenteric artery syndrome (SMAS) depending on the duration of the disease.
4.1. 1st group
Acute development of symptoms was in the stressful conditions, which in the catabolic stage are accompanied by significant weight loss. This is especially often observed in adolescents after surgery on the spine (6, 7). It is known that stress states are accompanied by a decrease in the pH of gastric 10 contents. In such cases, even high doses of proton pump inhibitors do not have an effect in improving gastric pH (14). Conservative treatment in conditions of partial obstruction of the duodenum was successful in 89% of patients after 2−59 (13.4 ± 2.9) days. Obviously, during this time, neither intravenous hyperalimentation, nor tube introduction of food into the small intestine could change either the total weight of patients or the volume of adipose tissue in the AMA. Moreover, after the restoration of patency in the duodenum, the distance between the vessels does not change (9). Therefore, the positive effect of conservative treatment is not associated with an increase in adipose tissue in the AMA, which does not support the hypothesis of the role of AMA in the pathogenesis of SMAS.
4.2. 2nd group
In patients of the 2nd group with functional dyspepsia, postprandial syndrome, and other peptic syndromes, the pathology is due to the hypersecretions of hydrochloric acid. They, in contrast to the patients in the 1st group, had a long history of the disease. Disturbance of the duodenal patency increased gradually: from slight disruption to complete obstruction. In these patients, as Ochsner showed, hypertrophy of the circular muscle layer of the third part of the duodenum distal to the sphincter of Oddi occurs. This may be the reason for the lower effectiveness of the short-term conservative treatment in patients of the 2nd group. As can be seen from Table 1, surgeons operated on the patients of the 2nd group, only based on a decrease in AMA (<25), or the distance between the aorta and SMA (<8 mm). In 47% of cases, the operation was performed without attempting conservative treatment. Conservative treatment was successful in 39% of cases, and in 14% of cases conservative treatment was short-term and ineffective. These patients were operated on. Thus, 61% of patients with peptic problems accompanied by duodenal dyskinesia were operated on only because they were thin or had no effect after short-term non-pathogenic conservative treatment (15).
5. Conclusion (Etiology, pathogenesis, diagnosis, and treatment of Ochsner‘s sphincter dyskinesia)
5.1. The above evidence leads to the following conclusions
1. The zone of contraction in the 3rd part of the duodenum with a length of 2.5−4.6 (3.30 ± 0.15) cm cannot be caused by compression of the intestine in the AMA, firstly, because between the aorta with a diameter of 2 cm and the SMA with a diameter of 0.5 cm, the length of the compressed intestine cannot be more than 1 cm. Secondly, the beginning of the contracted segment of the duodenum is a few centimeters cranial to the AMA. In length (3.2 ± 0.15 cm) and localization, the contracted segment of the duodenum corresponds to the sphincter Ochsner, which normally contracts to prevent an aggressive low pH bolus from entering the small intestine. A prolonged contraction of the sphincter Ochsner, which disrupts the passage into the jejunum, indicates its dyskinesia.
2. Article 8. Biank and Werlin (8) identifying AMA < 25 and distance between aorta and SMA < 8 mm as proof of SMAS is based on false information and is untrue. These parameters are proportional to BMI. Surgeons relying on these parameters operate on patients with dyspepsia, duodenitis, or duodenal ulcer without signs of obstruction. They produce a bypass duodenojejunostomy, because of which the aggressive chyme immediately from the stomach enters the jejunum, which inevitably leads to chronic enteritis.
3. The notion that the cause of SMAS is the loss of adipose tissue in the AMA and hyperalimentation over several weeks can lead to fat accumulation in AMA and to recovery does not withstand scientific scrutiny.
5.2. Etiology
Hypersecretion of hydrochloric acid are provoked by the food ingredients. These provocateurs or stimuli delayed onset diseases, that can be mediated by intestinal mucosal mechanisms involving not only IgE but also T cells, mast cells, and eosinophils that produce proinflammatory mediators. “Atopic dermatitis, celiac disease, or eosinophilic GI diseases, such as esophagitis, gastritis, gastroenteritis, enterocolitis and proctitis” belong to this kind of disease (16). It turned out that under stress “mast cells (MC) are important effectors of brain–gut axis that translate the stress signals into the release of a wide range of neurotransmitters and proinflammatory cytokines, which may profoundly affect the gastrointestinal physiology” (17). In both cases, one of the end products is acid hypersecretion. The lactose is the most common provocateur of hydrochloric acid hypersecretion (18). It causes the release of histamine from mast cells, which, through a chain of mediators, leads to the hypersecretion of hydrochloric acid. There are patients with histamine intolerance (19) and others. Thus, the hypersecretion of hydrochloric acid is the cause of all acid-dependent diseases (esophagitis, GERD, stomach ulcer and gastritis, duodenal ulcer and duodenitis, sphincter of Oddi dyskinesia and cholecystitis, as well as SMAS). As a rule, one of these problems, to a greater or lesser extent, is present in every patient with Ochsner’s sphincter dyskinesia.
5.3. Pathogenesis
Prolonged duodenal irritation with low-pH hydrochloric acid causes an increase in the tone of Ochsner’s sphincter, which gradually leads to wall hypertrophy, as Ochsner saw during the operation. If normally a fleeting contraction of the sphincter Ochsner is recorded as a pendulum movement of the bolus, then with dyskinesia, a narrowing of the intestine is clearly recorded. However, it is necessary to distinguish between the contraction of the sphincter Ochsner without disturbing the bolus evacuation into the jejunum, with obstruction of the duodenum, which is characterized by the expansion of the stomach and duodenum and is accompanied by vomiting.
5.4. Diagnosis
As shown above, the magnitude of the AMA and the distance between the aorta and the SMA are proportional to the BMI. This means that a decrease in these parameters only indicates the thinness of patients. Therefore, their definition on CT, MRI, and US does not make any sense. Two clinical conditions must be distinguished: (a) For symptoms of intestinal obstruction that are accompanied by recurring vomiting, a radiography is shown to determine the level of obstruction and the size of the stomach and duodenum. (b) Abdominal pain, weight loss, and other symptoms of acid-related disease require a barium x-ray. If sphincter Ochsner dyskinesia is detected, gastroscopy should be the next step to rule out other acid-related disorders and insertion of an enteral feeding catheter.
5.5. Treatment
Based on our hypothesis, dyskinesia of the sphincter Ochsner is caused by hypersecretion of hydrochloric acid and the pathogenetic treatment should be aimed at reducing the hydrochloric acid release and removing it from the duodenum. As the literature analysis has shown, the most effective are nasogastric decompression with nasojejunal feeding. In patients without obstruction of the duodenum, the use of PPI 40 mg twice a day for 4 weeks is justified. In patients with obstruction of the duodenum, the high-dose PPI therapy (intravenous loading dose followed by continuous infusion) (20, 21) is justified until the sphincter Ochsner is fully opened, followed by a transition to oral PPI reception.
The duodenal irrigation with alkaline solution is theoretically justified. In the absence of the effect of drug treatment to relieve spasm of the sphincter Ochsner, the use of Botox injections is justified. With sphincter Ochsner dyskinesia, there is no place for surgical treatment.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
References
1. van Beek A, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. (2017) 18:68–85. doi: 10.1111/obr.12467
2. Ochsner A. VIII. Construction of the Duodenum below the entrance of the common duct and its relation to disease. Ann Surg. (1906) 43:80–7. doi: 10.1097/00000658-190601000-00009
3. Aldot G, Kapandji M, Ringendach J. Physiology of timed duodenal intubation. II. Role of Ochsner’s sphincter in the mechanism of normal duodenal intubation and in that of certain prolongations of so-called 13 Oddi’s closed time. Arch Mal Appar Dig Mal Nutr. (1956) 45:449–57.
4. Levin M. Ochsner’s sphincter dyskinesia is the cause of superior mesenteric artery syndrome. J Gastrointest Surg. (2019) 23:1714–6. doi: 10.1007/s11605-019-04246-5
5. Levin M, Korshun Z, Mendelson G. [Duodenal motility in norm and in some diseases. Hypothesis]. Ter Arkh. (2016) 88:68–74. doi: 10.17116/terarkh201688468-74
6. Levin M. Sphincter Ochsner dyskinesia as a cause of superior mesenteric artery syndrome. Vestn Rentgenol Radiol. (2016) 97:110–7. doi: 10.20862/0042-4676-2016-97-2-110-117
7. Levin M. Pathogenetic significance of dyskinesia of the sphincter of oksner in the development of the syndrome of the superior mesenteric artery. Eksp Klin Gastroenterol. (2016) 12:67–72.
8. Biank V, Werlin S. Superior mesenteric artery syndrome in children: a 20- year experience. J Pediatr Gastroenterol Nutr. (2006) 42:522–5. doi: 10.1097/01.mpg.0000221888.36501.f2
9. Santer R, Young C, Rossi T, Riddlesberger M. Computed tomography in superior mesenteric artery syndrome. Pediatr Radiol. (1991) 21:154–5. doi: 10.1007/BF02015638
10. Neri S, Signorelli S, Mondati E, Pulvirenti D, Campanile E, Di Pino L, et al. Ultrasound imaging in diagnosis of superior mesenteric artery syndrome. J Intern Med. (2005) 257:346–51. doi: 10.1111/j.1365-2796.2005.01456.x
11. Eisenberg RL. Diagnostic imaging in internal medicine. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson J, Loscalzo J editors. Harrison’s Manual of Medicine. McGraw-Hill, Inc (2016). 694 p. *editm year.
12. Bhagirath Desai A, Sandeep Shah D, Jagat Bhatt C, Umesh Vaishnav K, Salvi B. Measurement of the distance and angle between the aorta and superior mesenteric artery on CTScan: values in Indian population in different B MI categories. Indian J Surg. (2015) 77, (Suppl. 2):614–7. doi: 10.1007/s12262-013-0941-1
13. Alzerwi N. Predictors of superior mesenteric artery syndrome: evidence from a case-control study. Cureus. (2020) 12:e9715. doi: 10.7759/cureus.9715
14. Lenz K, Buder R, Firlinger F, Lohr G, Voglmayr M. Effect of proton pump inhibitors on gastric pH in patients exposed to severe stress. Wien Klin Wochenschr. (2014) 127:51–6. doi: 10.1007/s00508-014-0637-y
15. Ganss A, Rampado S, Savarino E, Bardini R. Superior Mesenteric Artery Syndrome: a Prospective Study in a Single Institution. J Gastrointest Surg. (2019) 23:997–1005. doi: 10.1007/s11605-018-3984-6
16. Cuomo R, Andreozzi P, Zito F, Passananti V, De Carlo G, Sarnelli G, et al. Irritable bowel syndrome and food interaction. World J Gastroenterol. (2014) 20:8837–45. doi: 10.3748/wjg.v20.i27.8837
17. Konturek P, Brzozowski T, Konturek S. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. (2011) 62:591–9.
18. Minenna M, Palieri A, Panella C, Ierardi E. Gastro-oesophageal reflux disease and lactose malabsorption: casual comorbidity or neglected association? Dig Liver Dis. (2006) 38:437–8. doi: 10.1016/j.dld.2006.01.013
19. Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués M, Latorre-Moratalla M, Vidal-Carou M. Histamine intolerance: the current state of the art. Biomolecules. (2020) 10:1181. doi: 10.3390/biom10081181
20. Barkun A, Almadi M, Kuipers E, Laine L, Sung J, Tse F, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. (2019) 171:805–22. doi: 10.7326/M19-1795