Case summary
A 40-year-old female patient, a teacher, presented with a progressively increasing occipital headache with double vision for a week with nausea and mild instability while walking for the last 2 days. On examination, she was conscious, oriented, and obeying commands. There was early papilledema on fundus examination, with upgaze restriction, light near dissociation, and convergence retraction nystagmus. Her MRI imaging showed a heterogeneously enhancing dumbbell-shaped well-circumscribed, solid/cystic lesion measuring 2.3 cm × 2 cm × 3.1 cm [Transverse (TR) × anteroposterior (AP) × cephalocaudal (CC)] at the pineal region, causing extrinsic compression over the splenium of the corpus callosum with edema around, inferiorly abutting the tectal plate along the posterior aspect of the aqueduct of Sylvius with upstream mild dilatation of the third and lateral ventricles (Figure 1). A high-grade papillary parenchymal tumor was suspected.

Figure 1. Pre-op MRI images. (A) Sagittal, (B) Coronal and (C) Axial, MRI brain T2W images showing a well-circumscribed heterogeneous mass lesion situated in the pineal region with solid and cystic areas with extension to the splenium of the corpus callosum, splitting the tectum and causing compression to the aqueduct anteriorly with moderate obstructive hydrocephalus. (D) T1W contrast MRI brain images showing a well-circumscribed tumor with heterogeneous contrast enhancement (see arrows). (E) T2 FLAIR axial. (F) T2 FLAIR coronal.
She was planned for tumor excision and biopsy confirmation. Poppen’s occipital transtentorial approach was planned because the superior portion of the tumor was situated near the tentorium angle. She underwent gross total excision of the lesion. The tumor had both cystic and solid components, was greyish-white in color, and was moderately vascular. The cystic part was greyish-yellow, mucinous, and slimy but soft and suckable. The solid part was gritty, firm to hard, and papillary, which was difficult to dissect. The tumor capsule was adherent to the tectal plate and the third ventricular outflow tract (Video 1).
| Video 1. Video showing rare desmoplastic myxoid tumor of pineal region. https://youtu.be/SoB5VGsPPsQ |
Postoperatively she did well and did not have any new neurological deficits.
The histopathology report favored pineal myxoid epithelioid neoplasm with papillary features with moderate cellularity, composed of spindled and epithelioid cells within a collagenized matrix with myxoid changes.
Mitotic activity is low (0 mitoses/10 HPF), and tumor necroses were absent (Figure 2 as below).
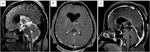
Figure 2. Histopathology and immunohistochemistry (IHC). (A and B) showing moderate cellularity, composed of spindled and epithelioid cells within a collagenized matrix with myxoid changes. Mitotic activity is low (0 mitoses/10 HPF), and tumor necroses were absent.
Further studies by IHC showed INI1 loss, CD34 positive, alpha-smooth muscle actin (SMA) was variably positive, and epithelial membrane antigen (EMA) was patchy positive on tumor cells. It was negative for OLIG2, CRX, GFAP, panCK, desmin, and synaptophysin, with a KI index was around 6%. Tumor cells show a convincing loss of nuclear SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)/INI1 expression and are positive for CD34 and EMA. The differential diagnosis was atypical teratoid/rhabdoid tumor (AT/RT).
Tumor cells showed a convincing loss of nuclear SMARCB1/INI1 (C) expression (Figure 3) and were positive for CD34 (D) and EMA (E). The Ki-67/MIB1 (F) proliferation index is around 6%. DNA methylation testing was recommended as it helps in subclassifying the SMARCB1 tumors. The final impression was SMARC B1 mutant neuro-epithelial desmoplastic myxoid tumor (DMT) of the pineal region.
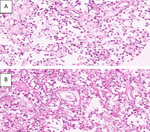
Figure 3. Tumor cells show convincing loss of nuclear SMARCB1/INI1 (A) expression and are positive for CD34 (B) and EMA (C). The Ki-67/MIB1 (D) proliferation index is around 6%.
The case was discussed in the tumor board. Due to the rarity of this neoplastic entity and having very little data on its biological behavior and long-term prognosis, adjuvant cranio-spinal radiation therapy was recommended. She successfully completed cranio-spinal radiation therapy for 6 weeks. She is doing well after 12 months of follow-up post-surgery.
Discussion
Desmoplastic myxoid tumor, SMARCB1 mutant, is a recently described but very rare yet distinct pineal parenchymal tumor seen in adults, according to the new WHO 2021 classification (1). Predominantly seen in adolescents and older individuals with a mean age of presentation at 36.6 years (average 15–61 years) with a slight preponderance to females (M:F, 4:6) (2) (Figure 4). Pineal region tumors are highly heterogeneous histologically and account for less than 1% of all intracranial neoplasms in adults and approximately 3–11% of pediatric brain neoplasms (3).

Figure 4. Post-op MRI images. (A) Sagittal T2W, (B) Axial and (C) Sagittal Immediate Post-op contrast MRI brain images showing gross total excision of the lesion with reduction in size of the ventricles.
The clinical behavior is not very well known, as only very few cases have been reported in the literature. Pineal region masses usually but not invariably cause syndromes of mass effect due to compression of the tectum and aqueduct of Sylvius, leading to obstruction to cerebro-spinal fluid (CSF), causing features of raised ICT with headache, nausea, vomiting, and variable eye symptoms like blurring of vision or diplopia. There may be compressive hypothalamic syndromes, such as diabetes insipidus and stunted growth in children. Occasionally patients present with classical Parinaud syndrome, which is an eponymous constellation of upward gaze palsy, convergence retraction nystagmus, light-near dissociation, and bilateral lid retraction.
Neuroimaging, even though challenging due to the varieties of tumors in the region, is essential for initial diagnosis and follow-up (1). MRI remains the investigation of choice for diagnosis (4, 5), treatment planning, and follow-ups. MRI best reveals lesion characterization, extent and spread of lesions, presence of suprasellar extensions, leptomeningeal, and cranio-spinal assessments in both adults and children. A CT scan is useful at best to find out the degree of ventricular dilatation, calcifications, or bleeding.
Pathologically, SMARCB1 gene (5), which is also known as INI1 (integrase interactor 1) and Hsnf5 (SNF5 homolog), is a matrix-associated actin-dependant regulator of chromatic which acts as a tumor suppressor. A new category of central nervous system (CNS) tumor accompanying an SMARCB1 mutation, i.e., DMT, SMARCB1-mutant was proposed by Christian Thomas et al. (6), which is located at the pineal region and seen in adolescents to older individuals with low-grade morphology. These tumors showed an admixture of variably dense cords of oval to spindled and epithelioid cells embedded in a heavily collagenized pale basophilic myxoid matrix. Loss of function of SMARCB1/INI1 is related to various SMARCB1-deficient tumor formations, the most common being the AT/RT in the pediatric age (7) group. Histologically, AT/RTs are characterized by rhabdoid cells in addition to diverse histological findings such as primitive neuroectodermal, mesenchymal, and epithelial components with varying IHC reactivity, high mitotic index, and necrosis associated with poor prognosis. They are embedded in the collagenized stroma with a myxoid matrix and have a low mitotic index and no necrosis with a better prognosis compared to AT/RT (6, 7). Histopathology with IHC will help in classifying them into various SMARCB1 mutant tumors. DNA methylation profiling will help to further sub-classify the SMARCB1 tumors of the CNS (5). DMT, SMACRB1 mutant predominantly occurs in adulthood, characteristically in the pineal region, showing bland morphological features, low mitotic activity, and hardly tumor necrosis (8).
Surgery is the treatment of choice (1). It can be just a shunt procedure for CSF diversion, an endoscopic biopsy, or an open surgical procedure aiming at subtotal or gross total excision. Surgical mortality is less than 3%, but morbidity may reach 20%, particularly in cases with extensive spread to the tectum (1). The choice of a surgical corridor will be based on the tumor extensions and their relation with the Vein of Galen and other deep venous complexes (9). The suboccipital transtentorial approach is preferable for tumors extending upward and displacing the venous complex inferiorly. The infratentorial supra cerebellar approach (10) utilizes the natural corridor for tumor reach but is better suited for lesions developing inferior to the venous complex. One study published in journal of neurosurgery (JNS) recommends a Supracerebelar Intratentorial approach for tumors in the midline and with extension to the third ventricle and an Occipital transtentorial approach for lesions extending to the fourth ventricle (11).
Clinical behavior, histological grading, and other diagnostic criteria of SMARCB1-mutant DMT of the pineal region are still evolving (1). Aggressive (grade 3) or recurrent tumors are subjected to adjuvant RT (1), whereas grades 1 and 2 tumors are closely observed. Presently the role of adjuvant radiotherapy remains unclear, irrespective of their biology. A curative intent cranio-spinal radiation is often prescribed by some authors (3). Prognostically, even though they may have a short clinical course, DMT, and SMARCB1-mutant may have a better prognosis compared to AT/RT, as they are found to be less aggressive. In one study, with a median follow-up of approximately 30 months, 30% of patients were dead (3), but the remainder were alive with stable disease or no signs of recurrence after subtotal resection followed by curative-intent craniospinal irradiation with an RT boost to the primary tumor region. Gross total resection (GTR) is the only consistent treatment-related prognostic factor (12). However, currently, due to the rarity of DMT SMARCB1, there is no consensus on optimal therapeutic strategies or standard characterization of the tumor’s behavior at present.
Conclusion
To conclude, this case report highlights the new tumor entity and aims to add to the growing body of knowledge about its clinical management. Our knowledge of the variety of SMARCB1/INI1-deficient tumors is slowly progressing, which includes the highly malignant AT/RT to less malignant DMTs. DMT, SMARCB1-mutant seems to have a better prognosis with long-term survival compared to their dreaded counterparts. However, careful attention is necessary because SMARCB1/INI1 deficiency is generally a genetic signature for concern. Armed with better diagnostic capabilities, we hope to identify such rare tumors more often and adopt measures for optimal management.
References
1. Lombardi G, Poliani PL, Manara R, Berhouma M, Minniti G, Tabouret E , et al. Diagnosis and treatment of pineal region tumors in adults: a EURACAN overview. Cancers (Basel) (2022) 14(15):3646. doi: 10.3390/cancers14153646
2. Manoranjan B, Omar AT, Wu HB, Nordal R, Starreveld YP. Clinical management of desmoplastic myxoid tumor, SMARCB1-mutant. Neuro Oncol (2022) 24(5):847–8. doi: 10.1093/neuonc/noac016
3. Favero G, Bonomini F, Rezzani R. Pineal gland tumors: a review. Cancers (Basel) (2021) 13(7):1547. doi: 10.3390/cancers13071547
4. Solomou AG. Magnetic resonance imaging of pineal tumors and drop metastases: a review approach. Rare Tumors (2017) 9(3):6715. doi: 10.4081/rt.2017.6715
5. Manoranjan B, Starreveld YP, Nordal RA, Dunham C, Bens S, Thomas C , et al. Desmoplastic myxoid tumor of pineal region, SMARCB1-mutant, in young adult. Free Neuropathol (2021) 2:2–14. doi: 10.17879/freeneuropathology-2021-3340
6. Thomas C, Wefers A, Bens S, Nemes K, Agaimy A, Oyen F , et al. Desmoplastic myxoid tumor, SMARCB1-mutant: clinical, histopathological, and molecular characterization of a pineal region tumor encountered in adolescents and adults. Acta Neuropathol (2020) 139(2):277–86. doi: 10.1007/s00401-019-02094-w Epub 2019 Nov 16.
7. Matsumura N, Goda N, Yashige K, Kitagawa M, Yamazaki T, Nobusawa S , et al. Desmoplastic myxoid tumor, SMARCB1-mutant: a new variant of SMARCB1-deficient tumor of the central nervous system preferentially arising in the pineal region. Virchows Arch (2021) 479(4):835–9. doi: 10.1007/s00428-020-02978-3 Epub 2021 Jan 9.
8. Wang YE, Chen JJ, Wang W, Zhang AL, Zhou W, Wu HB. A case of desmoplastic myxoid tumor, SMARCB1 mutant, in the pineal region. Neuropathology (2021) 41(1):37–41. doi: 10.1111/neup.12695 Epub 2020 Sep 9.
9. Behari S, Garg P, Jaiswal S, Nair A, Naval R, Jaiswal AK. Major surgical approaches to the posterior third ventricular region: a pictorial review. J Pediatr Neurosci (2010) 5(2):97–101. doi: 10.4103/1817-1745.76093
10. Katyal A, Jadhav A, Katyal A, Jagetia A, Bodeliwala S, Singhal GD , et al. Occipital transtentorial approach for pineal region lesions: addressing the controversies in conventional teaching. Surg Neurol Int (2021) 12:503. doi: 10.25259/SNI_168_2021
11. Cavalheiro S, Valsechi LC, Dastoli PA, Nicácio JM, Cappellano AM, Saba da Silva N , et al. Outcomes and surgical approaches for pineal region tumors in children: 30 years’ experience. J Neurosurg Pediatr (2023) 32(2):184–93. doi: 10.3171/2023.3.PEDS22468
12. Liu C, Carmicheal J, Baine MJ, Zhang C. Radiation therapy for pineal parenchymal tumor of intermediate differentiation: a case series and literature review. J Cent Nerv Syst Dis (2023) 15:11795735231160036. doi: 10.1177/11795735231160036
© The Author(s). 2025 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.